Limitations of The Octet Rule
Octet rule becomes relevant when considering main-group elements of the second and third periods in the periodic table, viz.: Carbon, nitrogen, oxygen, and fluorine. While the octet rule is very useful in terms of what might be expected from the behavior of many elements, there are limitations. Thus, there exist exceptions or cases when the octet rule just fails to work, making chemical bonding much more complex. Such examples are hydrogen and helium, known to be stable with fewer than eight electrons. Some atoms also can have more than the octet in so-called hypervalent molecules, which is an antipode of a simplistic view of electron configuration.
This Story also Contains
- Understanding Octet Rule
- Exceptions to the Octet Rule
- Supplement Your Knowledge
- Some Solved Examples
- Practice More Questions From the Link Given Below:
- Summary
In this article, we will cover the limitations of octet rule. This concept falls under the broader category of Chemical Bonding and Molecular Structure, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read -
Understanding Octet Rule
The octet rule suggests that atoms bond in a way that enables them to achieve a full outer shell of eight electrons for each atom hence offering them stability. This is best applied to main group elements, particularly to the second and third periods of the periodic table. These are done by three main mechanisms: ionic bonding, wherein electrons are transferred from atom to atom; covalent bonding, where they will be shared; and coordinate covalent bonding, in which both electrons come from one of the participating atoms.
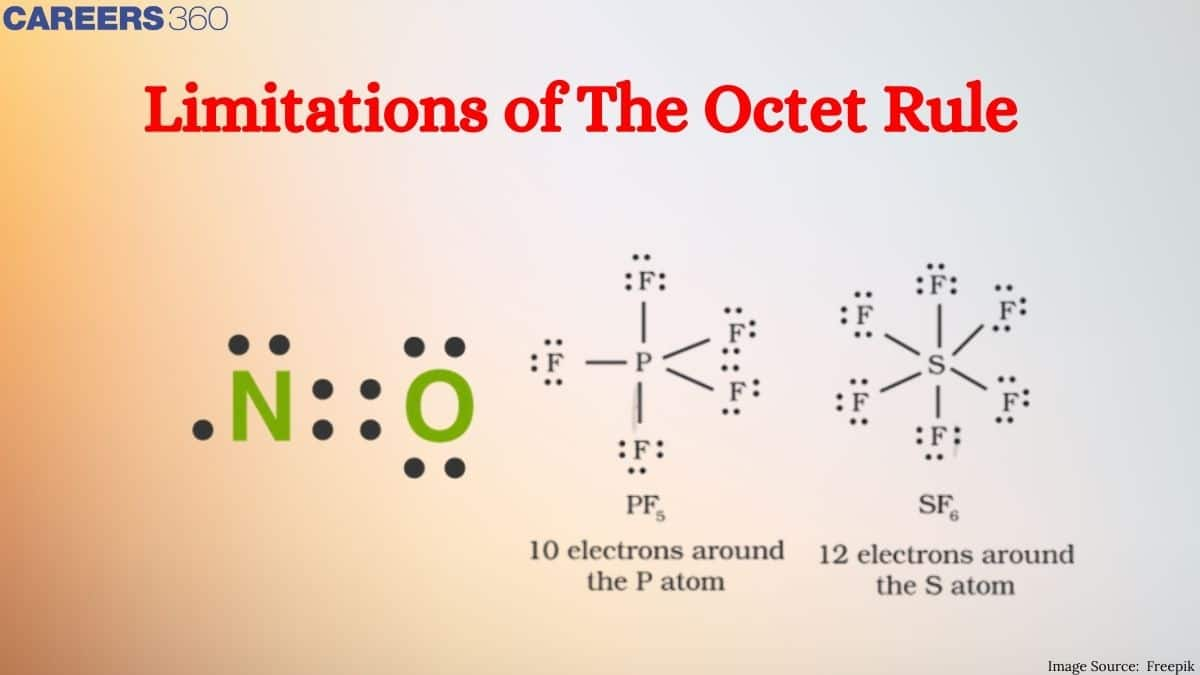
While the octet rule can be applied to explain a good number of chemical reactions, it is not universal. For example, certain elements are stable with fewer than eight electrons. Other atoms can hold more than an octet. Molecules such as these are called hypervalent. Examples include SF₆ and PF₅. The octet rule is a guiding principle but one that falls way short of describing the full complexity of chemical bonding and molecular stability.
Also Read:
Exceptions to the Octet Rule
Although octet rule is able to explain the formation of a large number of compounds, there are some exceptions to this rule as follows:
- Formation of compounds involving hydrogen. As hydrogen atom has only one shell containing one electron, it needs one more electron to complete its shell, i.e., to acquire the nearest noble gas configuration of helium. Hence, hydrogen needs to complete its duplet and not octet.
- Formation of compounds like BeCl2, BF3, AlCl3 etc. In each of these molecules, the central atom (Be, B or Al) has less than 8 electrons, i.e., these are electron deficient compounds as shown below:
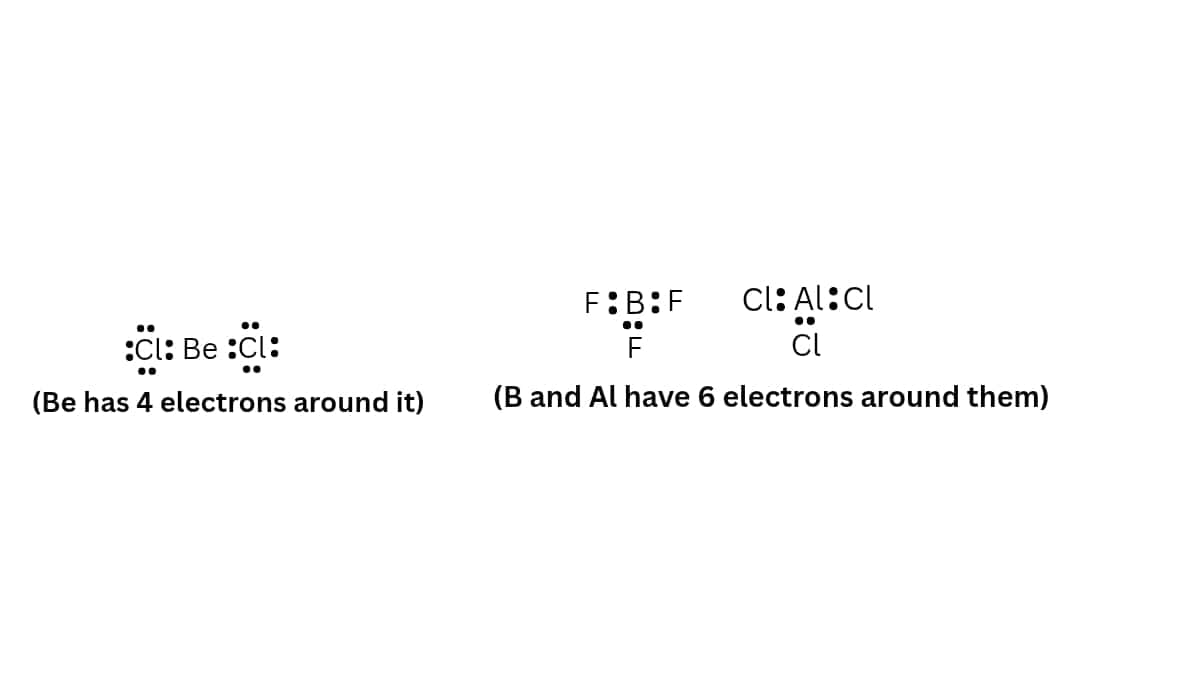 Thus, octet rule is violated.
Thus, octet rule is violated. - Formation of Compounds like PCl5, SF6, IF7, H2SO4 etc. In each of these molecules, the central atom has more than 8 electrons (expandes octet) as shown below:
.jpg) These compounds are called hypervalent compounds. Again the octet rule is violated in these molecules.
These compounds are called hypervalent compounds. Again the octet rule is violated in these molecules. - Formation of compounds of noble gases. Noble gases which have already complete octets (or duplet in case of helium) should not form compounds. However, their compounds like XeF2, XeF4, XeF6, KrF2 etc. have been actually prepared.
- Odd electron bonds/Odd electron molecules. There are some molecules and ions in which the bonded atoms contain odd number of electrons (usually 3) between them. The bonds thus present are called odd electron bonds and the molevules are called odd electron molecules. Some common examples are given below:
.jpg) A three electron bond is considered to be equivalent to half covalent bond.
A three electron bond is considered to be equivalent to half covalent bond.
Supplement Your Knowledge
Sugden's concept of singlet linkage. Sugden put forward the view that octet rule is never violated. He suggested that in case of molecules like PCl5, SF6, etc., some atoms are linked to the central atom by covalent bonds while others are linked by singlet bonds. A singlet bond is formed by one sided sharing of only one electron between the two atoms and is, therefore, represented by half arrow ($
\rightharpoonup
$) pointing from donor to acceptor. Thus, we have
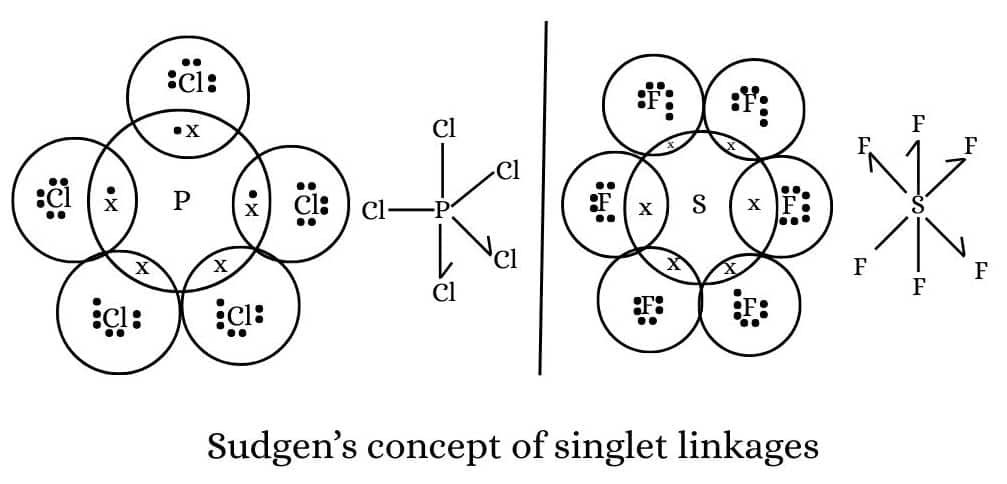
It can be seen that
No. of singlet bonds $=$ Total no. of bonds - No. of electrons required to complete the octet.|
Real-Life Applications and Importance
In real-life applications, the constraints of the octet rule realize themselves in several areas: from materials science to biochemistry and pharmacology. As one simple example, the development of pharmaceuticals is based on the idea of intermolecular interactions at the atomic level. For many biologically active compounds, the octet rule is not observed strictly; therefore, advanced theories like molecular orbital theory are needed to predict their behavior.
On the other side, new material creation in material science depends on manipulating atomic interactions. Properties in most modern materials, for example, semiconductors or catalysts, boil down indeed to electron-deficient/hypervalent species, which cannot be accounted for by the octet rule alone.
Related Topics:
Recommended topic video on ( Limitations of octet rule)
Some Solved Examples
Example 1:
Octet rule cannot be applied to the non-metals after:
1) Carbon
2) Silicon
3) Oxygen
4) Nitrogen
Solution:
The octet rule cannot be applied to the non-metals after silicon. These elements can “expand their octet” and have more than eight valence electrons around the central atom.
Hence, the answer is the option (2).
Example 2:
Octet rule is based upon:
1) Shape of the molecules
2) Chemical inertness of noble gases
3) Energy of the molecule
4) None
Solution:
The Octet rule is based on the chemical inertness of noble gases. This rule is derived from the fact that noble gases have a stable electron configuration with a full outer shell, influencing the bonding behavior of other elements.
Hence, the answer is the option (2).
Example 3:
The group having species with complete octets in their ionic form is:
1)$\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Na}, \mathrm{Mg}^{2+}$
2)$\mathrm{O}^{-}, \mathrm{F}^{-}, \mathrm{Na}^{+}, \mathrm{Mg}^{2+}$
3)$\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Na}^{+}, \mathrm{Mg}^{2+}$
4)$\mathrm{O}^{-}, \mathrm{F}^{-}, \mathrm{Na}, \mathrm{Mg}^{+}$
Solution:
The group having species with a complete octet in their ionic form includes O2-, F-, Na+, and Mg2+. These ions achieve a noble gas configuration with a complete octet.
Hence, the answer is option (3).
Example 4:
Which of the following is an example of an odd-electron molecule?
1) NO2
2) CO2
3) N2
4) O2
Solution:
Nitric oxide (NO) is an example of an odd-electron molecule because it has an odd number of valence electrons (11 in total).
Hence, the answer is the option (1).
Example 5:
The central atom in which of the following compounds is electron-deficient?
1) BeH2
2) BF3
3) CH4
4) NH3
Solution:
In BF3, the boron atom is electron-deficient because it only has six electrons around it instead of the full octet.
Hence, the answer is the option (2).
Example 6:
Number of molecules from the following which are exceptions to octet rule is_
$\mathrm{CO}_2, \mathrm{NO}_2, \mathrm{H}_2 \mathrm{SO}_4, \mathrm{BF}_3, \mathrm{CH}_4, \mathrm{SiF}_4, \mathrm{ClO}_2, \mathrm{PCl}_5$, $\mathrm{BeF}_2, \mathrm{C}_2 \mathrm{H}_6, \mathrm{CHCl}_3, \mathrm{CBr}_4$ [JEE Main 2024]
Solution:
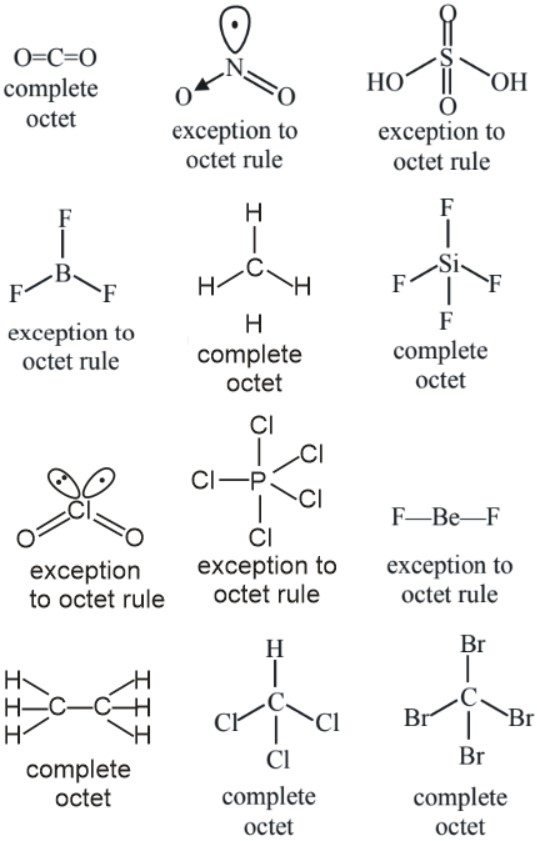
Hence, the answer is (6).
Practice More Questions From the Link Given Below:
Summary
The octet rule, in layman's terms, is one of the basic tenets of chemistry, guiding the formation of bonds between atoms to attain stability with a full outer shell of eight electrons. This paper has managed to point out some major limitations of the universal application of the octet rule. We have covered the concept, defining its tenets and mechanisms of bonding, but also its exceptions among incomplete octets, odd electron molecules, and hypervalent compounds.
Also Check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The octet rule says atoms tend to gain, lose, or share electrons so that they have eight electrons in their outer shell, like the noble gases. It applies well in many simple cases.
Some atoms, especially from the third period or beyond, can accommodate more than eight electrons in their valence shell (because of additional orbitals). This leads to compounds like PF₅, SF₆, etc., where the central atom has more than eight electrons.
No. The rule is useful for drawing Lewis structures, but it doesn’t predict shapes, bond angles, or relative stability/energy of molecules. Other theories (VSEPR, molecular orbital theory, etc.) are required for that.
Atoms like hydrogen, beryllium, boron, and aluminium often form compounds in which the central atom has fewer than eight electrons in its valence shell (electron‑deficient compounds).
These are species that have an odd number of valence electrons. Because of that, not all atoms can achieve a full octet. Examples include NO and NO₂.
Because elements in higher periods have access to d (and sometimes f) orbitals which allow more flexible electron arrangements. Also, some atoms simply don't have enough valence electrons to fill an octet, and some molecules have odd numbers of electrons.
No. The rule is useful for drawing Lewis structures, but it doesn’t predict shapes, bond angles, or relative stability/energy of molecules. Other theories (VSEPR, molecular orbital theory, etc.) are required for that.
If the central atom is in period 2, often the octet rule is followed.
If valence electrons are odd‑numbered, expect odd electron molecules.
If the central atom is in period 3 or beyond, or there are many bonding partners, expect possible expanded octet.
When formal charges become large or when resonance can reduce charge, alternative structures may be preferred.

