Bond Parameters - Bond Order, Angle, Length, and Energy
The so-called bond parameters describe the nature and the strength of a chemical bond in molecules. Among them, each one, such as bond order, angle, length, and energy, has its role in predicting the stability of compounds and the degree of reactivity. For example, the parameters of a bond might influence the design of new drugs or advanced materials or even the understanding of the biological process at the molecular level.
This Story also Contains
- Understanding Bond Parameters
- Relevance and Applications of Bond Parameters
- Some Solved Examples
- Practice More Questions From the Link Given Below:
- Summary
In this article, we will cover the concept of the Bond Parameters - Bond Order, Angle, Length, and Energy. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read -
Understanding Bond Parameters

Bond parameters are basic properties that describe covalent bonds between atoms. They include:
- Bond Order: The number of pairs of electrons shared between two atoms. The concept is quantifiable from a Lewis structure of a molecule and describes the strength and therefore stability of a bond. The greater the bond order, the greater the amount of bonding: triple bond is three, and single bond is one.

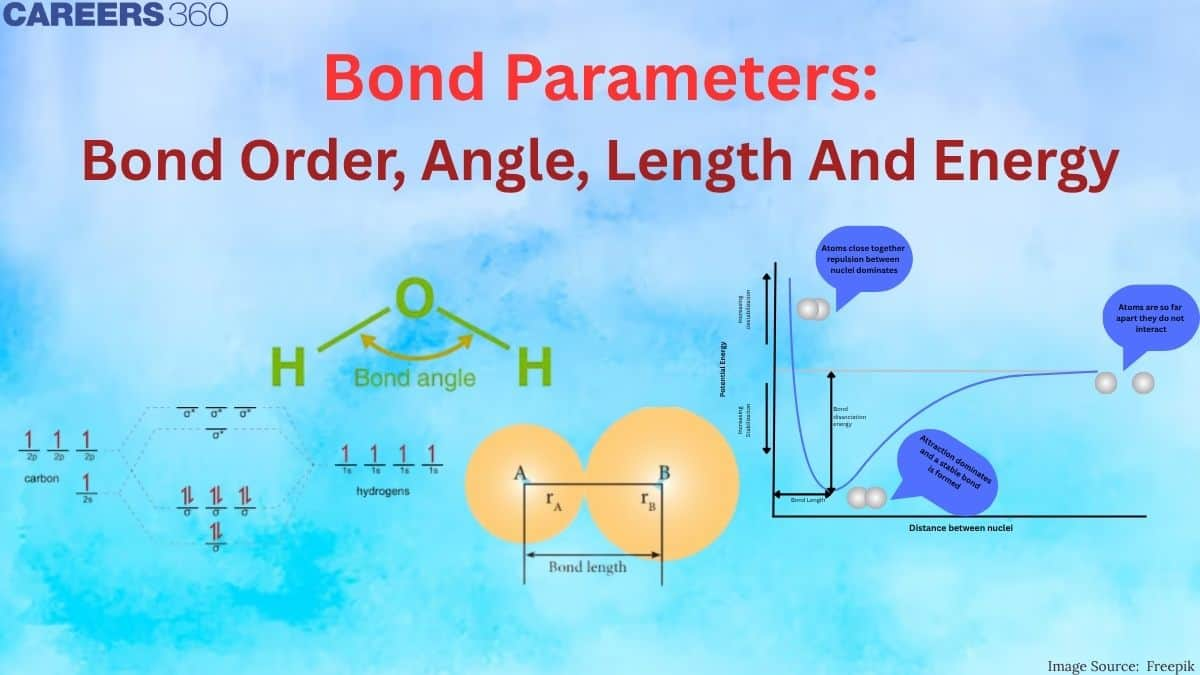
- Bond Angle: The angle formed by two covalent bonds sharing one common atom. It is a very important part in the determination of molecular geometry for compounds. For instance, the bond angle in water, H₂O, is approximately 104.5°, hence giving it some of its properties.

- Bond Length: The distance between the nuclei of two atoms bonded to one another. These vary in size depending on the type of bond. Triple bonds are shorter than double bonds, and these in turn are shorter than single bonds. The size of the bonded atoms and the bond order may be influential in determining the length.
- Bond Energy: This is the amount of energy needed to break a bond between atoms. As such, stronger bonds will exhibit higher bond energies, hence portraying the degree of stability of such bonds.
- All these parameters are concerned with giving information about the stability and reactivity of chemical compounds and thus feature centrally in understanding chemical behavior.
Aspects of Bond Parameters
Parameters of bonds can be classified into types, some of which include the following with their unique characteristics and implications:
1. Bond Order: By definition, it is the number of shared electron pairs involved in the bond. It can also be looked at from the view of molecular orbital theory, where sometimes the order is calculated as half of the number of electrons that are bonded minus antibonding. This concept comes in as major help while predicting the stability of the molecules.
2. Bond Angle: They can vary greatly depending on the molecular structure. For example, that of CH₄ is roughly about 109.5°, while that of CO₂ is 180°. These are very essential values in defining the molecular shape and reactivity.
3. Bond Length: This depends mostly on the type of atoms involved and their electronegativities. For instance, the bond length in a carbon-carbon single bond, C–C, is longer than in a carbon-carbon double bond, C = C.
4. Bond Energy: Probably at the top of the list of the most important factors in a chemical reaction is the bond energy. For instance, how much energy it would take to break bonds in a molecule could completely turn things around. The more force that holds atoms together—the stronger a bond—the more energy it takes to break, hence altering pathways and kinetics of reactions.
Each of these parameters contributes not only to an understanding of molecular structure but makes important contributions to predictions of chemical behavior.
Bond length
It is the distance between the nuclei of two bonded atoms when the lowest potential energy between the atoms is achieved.
Bond angle
It is the angle between any two covalent bonds that share a common atom as shown in the figure.

Bond distances (lengths) and angles are shown for the formaldehyde molecule, H2CO.
Bond energy
It is the energy required to break one mole of bonds between two atoms in a gaseous state. For example, the bond enthalpy of the H-H bond is 435.8 kJ/mol.
H2( g)→H(g)+H(g): ΔaH′=435.8k⋅mol−1
Bond Strength
It is the strength by which the bonded atoms are held together in a molecule. It is measured in terms of the bond energy, in other words, it is the amount of energy required to break the bond.
|
Average Bond Lengths and Bond Energies for Some Common Bonds | ||
|
Bond |
Bond Length (Å) |
Bond Energy (kJ/mol) |
|
C–C |
1.54 |
345 |
|
C=C |
1.34 |
611 |
|
C≡C |
1.20 |
837 |
|
C–N |
1.43 |
290 |
|
C=N |
1.38 |
615 |
|
C≡N |
1.16 |
891 |
|
C–O |
1.43 |
350 |
|
C=O |
1.23 |
741 |
|
C≡O |
1.13 |
1080 |
Also Read:
Relevance and Applications of Bond Parameters
These parameters of a bond have important implications both in the academic and practical application. Chemistry needs these parameters as basic in determining what nature the interaction the molecules will take in a chemical reaction. For instance, the bond order may indicate the stability of a molecule. This is important in designing new compounds in pharmaceuticals. Increased bond energy often goes with increased stability, a very important criterion for drugs.
Parameters of the bond are useful in biochemistry, illuminating the interactions between biomolecules. For example, bond angles and lengths of some enzymes may be responsible for their catalytic efficiency and thus their participation in metabolic pathways.
A bond parameter is therefore of importance in explaining the nature of chemical bonds and their applications in most scientific fields. The application goes as far as drug design, and materials science, to practices in theoretical and practical applications.
Related Topics:
Recommended topic video on ( Bond Parameters-Bond angle, Bond order, Bond length, and Bond energy)
Some Solved Examples
Example 1:
Arrange the following compounds in order of increasing C-halogen bond length CH3Cl, CH3Br, CH3I, and CH3F
1) CH3F < CH3Cl < CH3Br < CH3I
2)CH3F < CH3Br < CH3Cl < CH3I
3) CH3F < CH3I < CH3Br < CH3Cl
4)CH3Cl < CH3Br < CH3F < CH3I
Solution:
To determine the order of increasing C-halogen bond length, we need to consider the size of the halogen atoms. The larger the halogen atom, the longer the bond length.
The order of halogen size is:
I > Br > Cl > F
Hence, the correct order is:
CH3F<CH3Cl<CH3Br<CH3 (Option 1)
Example 2:
Arrange the compounds (O2),(H2O2), and O3 in order of increasing O-O bond length.
1) $\mathrm{H}_2 \mathrm{O}_2>\mathrm{O}_3>\mathrm{O}_2$
2) $\mathrm{H}_2 \mathrm{O}_2<\mathrm{O}_3<\mathrm{O}_2$
3) $\mathrm{H}_2 \mathrm{O}_2>\mathrm{O}_3<\mathrm{O}_2$
4) $\mathrm{O}_2>\mathrm{O}_3>\mathrm{H}_2 \mathrm{O}_2$
Solution:
Bond length decreases with the multiplicity of bonds. This is because a larger number of electrons shared between two atoms results in a greater attractive force between the electrons and the nuclei, leading to shorter bond lengths.
$\left(\mathrm{O}_2\right)$ has a double bond, which is shorter.
$\left(\mathrm{O}_3\right)$ has a bond order between a single and a double bond.
$\left(\mathrm{H}_2 \mathrm{O}_2\right)$has a single bond.
Thus, the correct order is:
$\left(\mathrm{H}_2 \mathrm{O}_2>\mathrm{O}_3>\mathrm{O}_2\right)$ (Option 1)
Example 3:
What happens to the bond angle as the S-character of a hybrid orbital decreases?
1) The bond angle decreases.
2) The bond strength increases.
3) The bond length decreases
4) Size of the orbital decreases.
Solution:
The bond angle decreases as the S-character of a hybrid orbital decreases. This is because:
The percentage of S-character is directly proportional to the bond angle.
A higher S-character means a larger bond angle.
When the S-character decreases, the bond angle also decreases.
Hence, as the S-character of a hybrid orbital decreases, the bond angle decreases.
The Correct answer is option 1
Example 4:
Why is the H-P-H angle in(PH3) smaller than the H-N-H angle in(NH3)?
1) Higher electronegativity of nitrogen.
2) More repulsion between lone pair and bond pair in NH3.
3) Lower electronegativity of nitrogen
4) None
Solution:
The H-P-H angle in(PH3) is smaller than the H-N-H angle in(NH3) because of the higher electronegativity of nitrogen compared to phosphorus.
Nitrogen has a higher electronegativity, which leads to greater electron density around nitrogen, reducing electron pair repulsion and resulting in a larger bond angle.
Phosphorus, being less electronegative, allows for more electron pair repulsion, resulting in a smaller bond angle.
Therefore, the H-N-H angle in(NH3) is greater than the H-P-H angle in(PH3).
The Correct answer is Option 1
Example 5:
Which molecule has the highest bond energy:
1) N-N
2) F-F
3) C-C
4) O-O
Solution:
To find the molecule with the highest bond energy, consider the following:
Single-bond energies tend to increase across a period.
The C-C bond is stronger than N-N, O-O, and F-F bonds because of the non-bonding electron repulsion in N-N, O-O, and F-F due to their small atomic sizes.
Given the single bonds, the C-C bond has the greatest bond energy because it is less affected by non-bonding electron repulsion.
The correct answer is: C-C (Option 3)
Example 6:
Consider the ions/molecule
$\mathrm{O}_2^{+}, \mathrm{O}_2, \mathrm{O}_2^{-}, \mathrm{O}_2^{2-}$
For increasing bond order the correct option is :
[JEE Main 2022]
1) $\mathrm{O}_2^{2-}<\mathrm{O}_2^{-}<\mathrm{O}_2<\mathrm{O}_2^{+}$
2) $\mathrm{O}_2^{-}<\mathrm{O}_2^{2-}<\mathrm{O}_2<\mathrm{O}_2^{+}$
3) $\mathrm{O}_2^{-}<\mathrm{O}_2^{2-}<\mathrm{O}_2^{+}<\mathrm{O}_2$
4) $\mathrm{O}_2^{-}<\mathrm{O}_2^{+}<\mathrm{O}_2^{2-}<\mathrm{O}_2$
Solution:
From MOT i.e molecular orbital Theory,
$\begin{aligned} & \text { Bond order }=\frac{N_b-N_a}{2} \\ & N_b=\text { number of bonding electrons. } \\ & N_a=\text { number of antibonding electrons. }\end{aligned}$
for $\mathrm{O}_2^{+}, \mathrm{N}_{\mathrm{b}}=10, \mathrm{~N}_{\mathrm{a}}=5$
$
\text { Bond order }=\frac{10-5}{2}=2.5
$
for $\mathrm{O}_2, \mathrm{~N}_{\mathrm{b}}=10, \mathrm{~N}_{\mathrm{a}}=6$
$
\text { Bond order }=\frac{10-6}{2}=2
$
for $\mathrm{O}_2^{-}, \mathrm{N}_{\mathrm{b}}=10, \mathrm{~N}_{\mathrm{a}}=7$
$
\mathrm{B} \cdot \mathrm{O}=\frac{\mathrm{N}_{\mathrm{b}}-\mathrm{N}_{\mathrm{a}}}{2}=\frac{10-7}{2}=1.5
$
for $\mathrm{O}_2^{2-}, \mathrm{N}_{\mathrm{b}}=10, \mathrm{~N}_{\mathrm{a}}=8$
$
\mathrm{B} \cdot \mathrm{O}=\frac{\mathrm{N}_{\mathrm{b}}-\mathrm{N}_{\mathrm{a}}}{2}=\frac{10-8}{2}=\frac{2}{2}=1
$
$\therefore$ Bond order : $\mathrm{O}_2^{2-}<\mathrm{O}_2^{-}<\mathrm{O}_2<\mathrm{O}_2^{+}$
Hence, the answer is the option (1).
Practice More Questions From the Link Given Below:
Summary
Bond order, bond angle, bond length, and bond energy are therefore very critical characteristics defining the nature and strength of a chemical bond. These parameters, related to molecular stability and reactivity, consequently affect an extremely wide range of areas in science: from chemistry and materials science to biochemistry. For a scientist to know the parameters of the bond means that they would be in a position to predict the chemical behavior and hence design new materials or develop pharmaceuticals with much greater chances of success.
Also Check
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Bond order is the number of chemical bonds between a pair of atoms (or an indicator of bond multiplicity).
In simple cases (Lewis structure), single = 1, double = 2, triple = 3.
In Molecular Orbital Theory: Bond order = (number of bonding electrons − number of antibonding electrons) / 2
Yes — polar bonds may have additional electrostatic (Coulombic) stabilizing/destabilizing effects.
But the basic strength is still determined by electron sharing (bond order & overlap).
So polarity is a modifying factor, not the primary determinant.
Single bonds (σ bonds) allow free rotation about the axis without breaking the bond.
In a double bond, the π component prevents free rotation (rotating would break the π overlap), so the geometry is locked.
Bond length is the average equilibrium distance between the nuclei of two bonded atoms.
It’s usually close to the sum of their covalent radii.
Measured by techniques like X‑ray diffraction, electron diffraction, spectroscopy.
Bond angle is the angle between two bonds sharing a common atom.
It determines the shape (geometry) of a molecule (using VSEPR, hybridization concepts).
Influencing factors: lone pairs, bond pair–bond pair repulsions, sizes of substituents, electronegativity differences, hybridization.
Higher bond order → shorter bond length and higher bond energy
Lower bond order → longer bond length and lower bond energy
(Because more shared electrons pull the nuclei closer and strengthen the bond)

