Classification of Elements in Modern Periodic Table - Meaning, Importance, FAQs
Why do some elements behave like metals while others show completely non-metallic character? Why does sodium react vigorously with water, whereas neon remains totally unreactive? Is there a logical pattern behind the chemical and physical properties of elements, or are they random? You will find these answers by reading this article on Classification of Elements in the Modern Periodic Table. The periodic table is an arrangement of elements according to their increasing atomic number and their chemical properties, as well as the order of their electronic configuration. The elements are arranged from top to bottom and from left to right in the periodic table. The table comprises 118 total elements. It is made up of 7 rows referred to as periods, as well as 18 columns referred to as groups.
This Story also Contains
- Modern Periodic Table
- Need for the Classification of Elements
- Classification of Elements in the Periodic Table
- Classification of Elements into Periods in the Periodic Table
- Classification of elements into groups in the periodic table
- Points to Remember :
- Some Solved Examples

Modern Periodic Table
In the Modern Periodic Table, Periodic properties of elements are determined by periodic functions of atomic numbers, according to the current periodic law. Across each row, elements were arranged from left to right in increasing order of their atomic numbers. There is a regular pattern in elements with similar properties.
Need for the Classification of Elements
As new elements are discovered, it becomes important to schedule as well as classify all existing elements. Physical as well as chemical properties, as well as atomic mass, were examined, therefore. Various researchers tried to describe elements based on chemical as well as physical characteristics. As the number of known elements increased, the study of their individual properties became difficult and confusing. Each element has its own physical and chemical characteristics, and learning them separately was neither systematic nor efficient. This created the need to classify elements in a logical and organized manner.
Classification of Elements in the Periodic Table
Periodic classification of elements class 11 is a method for classifying elements based on their features, in which we keep elements that are similar in one group and the rest of the elements in the other. Several empty spots have been left in the periodic table classification to put elements that will be discovered in the future without interrupting the elements' trending periodicity.
The current periodic table classification has been divided into seven periods (also known as horizontal rows) and eighteen groups (also known as vertical columns). The elements are grouped ascendingly according to their atomic numbers and the way electrons fill their atomic energy sublevels, according to the build-up hypothesis, with each element having one more electron than the one next to it.
The classification of elements in the periodic table in the contemporary periodic table classification has been done in such a way that the majority of the elements are metals. These metals are found on the periodic table classification's left side. Non-metals are a group of elements in the table that number less than 20 and are found on the right side of the table. Some of the elements that occur on the boundary between Metals and non-metals are known as metalloids. Metal and nonmetal identical properties are shown by these groups. Most of these elements are solids; just 11 of them, noble gases, oxygen, nitrogen, fluorine, chlorine, and hydrogen, exist as gases, and two of them, mercury and bromine, are liquids.
Also read :
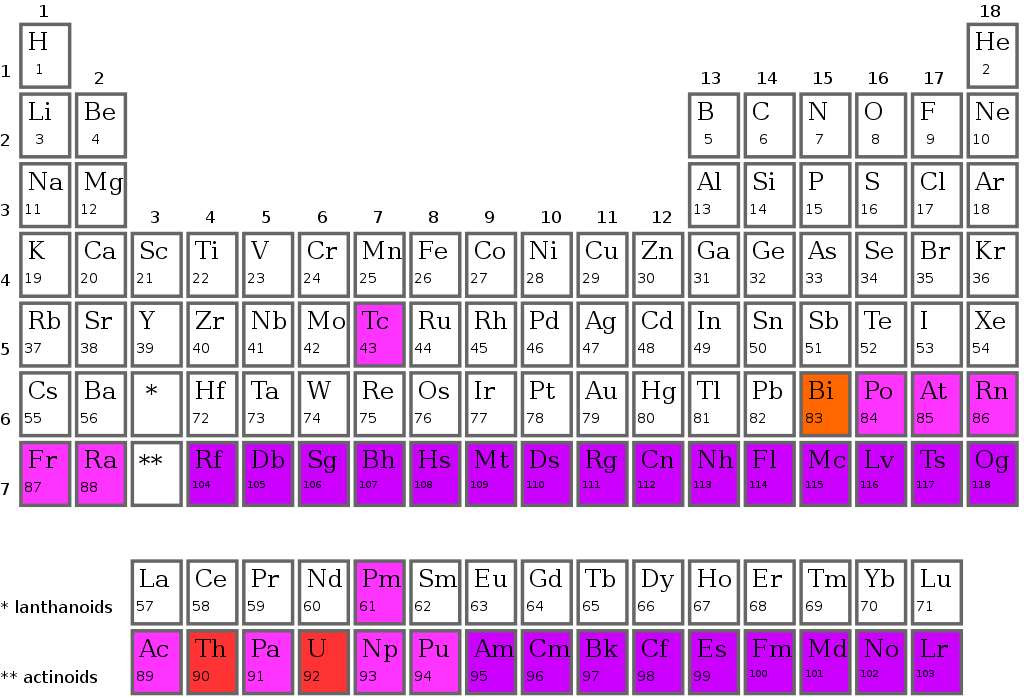
Classification of Elements into Periods in the Periodic Table
The periodic table's horizontal row is called a period. The periodic table is divided into seven periods, each starting on the far left. When a new primary energy level begins to fill with electrons, a new period begins. Period 1 only includes two elements (hydrogen and helium), but periods 2 and 3 each have eight. There are 18 elements in Periods 4 and 5.
Because the two bottom rows that are isolated from the rest of the table pertain to those periods, they have 32 elements. They're pulled out to make the table itself fit on a single page more readily.
Related Topics
Classification of elements into groups in the periodic table
Modern periodic tables have vertical columns called groups. In the periodic table, there are 18 groups. one through eighteen groups are numbered. The elements in each group have the same outer shell electronic configuration.
Based on the organization of the outer shell electrons, a group is a vertical column of the periodic table. There are 18 groupings in all. You should be familiar with both of the numerical schemes that are typically used to designate groups. The letters A and B are employed in the conventional system in the United States. 1A and 2A are the first two groups, while 3A through 8A are the last six. The name of the middle group is observed to begin with B. In Europe, however, there was a slightly different structure in place. To avoid confusion, the International Union of Pure and Applied Chemistry (IUPAC) agreed on a simple 1 through 18 method from left to right as the official system for numbering groups.
Points to Remember :
Periods (rows) and groups (columns) make up the modern Periodic Table (columns). There are 18 groups, each with a different number of periods ranging from one to seven.
- The energy level or orbit number is represented by a period.
- There are only two elements in the first period. This is the shortest time frame.
- Each of the second and third periods has eight elements. Small periods are what they're called.
- Each of the fourth and fifth periods has 18 elements. These are referred to as extended periods.
- Each of the sixth and seventh periods has 32 elements. These are referred to as "very long times."
- The number of valence electrons in an element is equal to its group number.
- Metals are accommodated on the left end while nonmetals are accommodated on the right end of the table.
- Noble elements are found in the periodic table's last group.
Also read -
Some Solved Examples
Question 1: What is the correct order of Calcium, Strontium, and Barium to satisfy Dobereiner’s law of triads?
1) Calcium, Barium, and Strontium
2) (correct) Calcium, Strontium and Barium
3) Strontium, Barium, and Calcium
4) Barium, Strontium, and Calcium
Solution:
DOBEREINER TRIAD RULE
J.W. Dorbereiner pointed out that within a group of three elements having similar chemical and physical properties, the atomic weight of the middle element is the mean of the other two. Some examples of such triads are given below. He also pointed out the triad - iron, cobalt, and nickel in which the atomic weights of the elements are almost the same.
Ca - 40 amu
$\mathrm{Sr}-88$ amu
Ba - 137 amu
$\frac{40+137}{2}=\frac{177}{2}=88.5$
Hence, the answer is option (2).
Question 2: Which of the following is most abundant in earth's crust?
1) (correct) O
2) Si
3) Al
4) Fe
Solution:
In the Earth's crust order of abundance of elements is
O > Si > Al > Fe
Hence, the answer is option (1).
Question 3: Which of the following statements is correct?
1) The Law of Triads was given by Newlands
2) Mendeleev’s periodic law was based on the atomic number of the elements
3) (correct) Law of octaves was given by Newlands
4) All of these
Solution:
NEWLAND'S LAW of OCTAVES
John Alexander Reina Newland in England made the first attempt to correlate the chemical properties of the elements with their atomic weight. According to him -
-
If the elements are arranged to thaccording eir increasing atomic weights, every eighth element has similar properties to the first one like the first and eighth notes in music. For example
|
-
Inert gases were not discovered till then.
-
All the elements could not be classified on this basis.
The law of triads was given by Dobereiner.
Mendeleev’s periodic law was based on the atomic masses of the elements.
The law of octaves was given by Newlands.
Hence, the answer is option (3).
Question 4: Identify the group that is not a Dobereiner triad:
1) Li, Na, K
2) (correct) Be, Mg, Cr
3) Ca, Sr, Ba
4) Cl, Br, I
Solution:
We know that according to Dobereiner's law of triads, the atomic mass of the middle element of a triad is the arithmetic mean of the atomic masses of the other two elements.
The group that does not follow Dobereiner’s law of triad is Be, Mg, Cr.
It is because Cr is not in the same group as Be and Mg. Be and Mg belong to group second whereas Cr belongs to group sixth.
Moreover, the arithmetic mean of the atomic masses of the other two elements is not equal to the atomic mass of the middle element.
Atomic mass of Be = 8, Mg = 24, Cr = 51.9
Arithmetic mean = 51.9+82=29.9. This arithmetic mean is not equal to the atomic mass of the central element.
Hence, the answer is option (2).
Frequently Asked Questions (FAQs)
Metals are elements that typically have high electrical conductivity, malleability, ductility, and a lustrous appearance. They are predominantly found on the left side and in the center of the periodic table, including groups 1 to 12 and some other groups.
Elements are classified into terms of their increasing atomic number in the modern periodic table.
The current periodic table has been divided into seven periods (also known as horizontal rows) and eighteen groups (also known as vertical columns). The elements are grouped ascendingly according to their atomic numbers and the way electrons fill their atomic energy sublevels
There are only two elements in the first period. This is the shortest period.
There were a total of 118 elements recognized by the International Union of Pure and Applied Chemistry. The first 94 elements are found naturally on Earth, whereas the remaining 24 are created in nuclear processes.
