Condensation - Definition, Examples, Process, FAQs
Condensation is a phenomenon we experience every day. Condensation is the process by which water vapor is converted into a gaseous form in the air. When the temperature is very high. There are small bunches of water on the mirror when you touch the mirror. How has this fog evolved? From where have these water beads come? That is Condensation. For example, when the bathroom cools down after a hot shower, the air falls, and the water vapor falls into the cold mirror. Thus, the water vapor condenses and forms liquid water on the mirror surface.
This Story also Contains
- Condensation
- Examples of Condensation
- Condensation Reaction
- Condensation in the Water Cycle
- Some Solved Examples
Condensation
- Condensation is the process of converting a gas into a liquid.
- The reaction in which two molecules combine and lose water is called condensation.
- Condensation is defined as removing heat from the system and turning steam into liquid.
Examples of Condensation
The Condensation examples are
- Clouds in the sky
- Visible breath in cold conditions.
- Fog in the air
Also read
| NCERT Solutions for Class 11 Chemistry | NCERT notes Class 11 Chemistry |
| NCERT Solutions for Class 12 Chemistry | NCERT notes Class 12 Chemistry |
| NCERT Solutions for All Subjects | NCERT Notes For All Subjects |
Condensation Reaction
The condensation reaction combines two molecules into one molecule, usually with the loss of a small molecule, such as water.
Basic Process of Condensation
Water condensation occurs when water transforms its phase from gaseous to liquid or crystalline form. Every gas can condense at high pressure and low temperature. The condensation process can technically take place at any temperature, as long as the gas's liquid State is below the condensing gas pressure. In the process of condensation, the molecules in matter slow down because heat energy is removed, which changes the matter into a solid state, that is, it causes changes in the three states of matter.
A condensation reaction is a chemical reaction that combines two molecules to form a larger one with a water molecule. Alcoholics and many other molecular reactions to condensation often occur in living organisms.
- The hydrogen and hydroxide taken from a condensation reaction originate from different molecules, while the H and OH removed come from the same molecule in the dehydration reaction.
- The H is removed from an electronegative atom in the condensing reaction, whereas the H is removed from a C atom in the dehydration reaction.
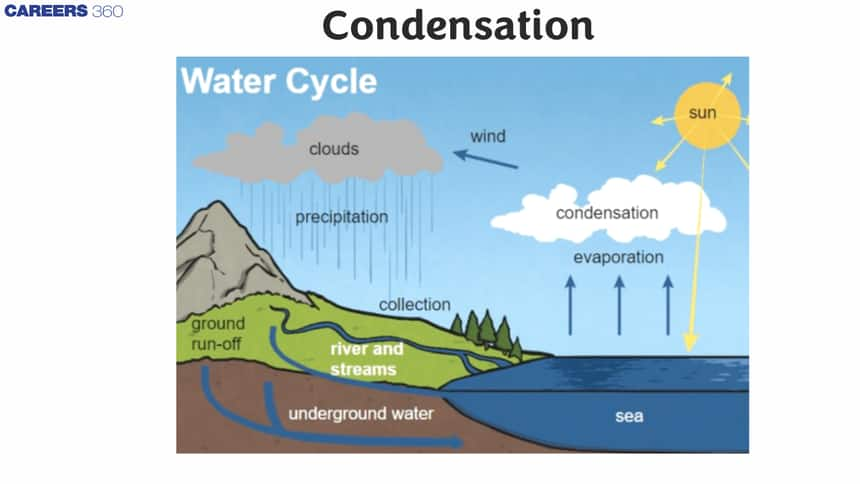
Condensation in the Water Cycle
- For the water cycle, condensation is important because it is responsible for cloud formation.
- Water vapor in the air causes the formation of clouds that eventually fall in the form of rain.
- The movement of water molecules is responsible for this phase of water change among solid, liquid, and gas.
- In vapor form, water molecules in relation to the fluid are arranged randomly.
- As condensation occurs, water molecules develop more organization, leading to the transfer of heat from the vapor state to the liquid state in the atmosphere.
- This usually occurs with warm air up and cool air falling in the atmosphere.
The atmosphere must be completely saturated to cause condensation (to reach maximum vapor pressure). Usually, the dust particles or smoke, or microscopic bacteria are condensed. The water cycle plays a very important role and thus helps to maintain the environmental water balance. Scientists and engineers also use it for the separation of mixtures and the production of pure materials in various industrial processes.
Also read-
Some Solved Examples
Question 1: Which of the following compounds will NOT undergo aldol condensation?
A. Acetaldehyde
B. Acetone
C. Benzaldehyde
D. Propanal
Solution:
Aldol condensation requires the presence of α-hydrogen.
- Acetaldehyde, acetone, and propanal contain α-hydrogens.
- Benzaldehyde lacks an α-hydrogen, and therefore, it does not undergo aldol condensation.
Hence, the correct answer is option (C).
Question 2: The major product formed when acetaldehyde undergoes aldol condensation, followed by heating, is:
A. Ethanol
B. 3-Hydroxybutanal
C. Crotonaldehyde
D. Butanal
Solution:
- First step: Aldol addition gives 3-hydroxybutanal
- On heating: dehydration occurs, forming an $\alpha, \beta$-unsaturated aldehyde
- Final product $=$ Crotonaldehyde $\left(\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CHO}\right)$
Hence, the correct answer is option (C).
Question 3: Which base is commonly used to carry out aldol condensation?
A. $\mathrm{NaBH}_4$
B. Dilute NaOH
C. Concentrated $\mathrm{H}_2 \mathrm{SO}_4$
D. $\mathrm{KMnO}_4$
Solution:
Aldol condensation is generally carried out in dilute alkali $(\mathrm{NaOH}$ or KOH$)$ which abstracts $\alpha$-hydrogen to form the enolate ion.
Hence, the correct answer is option (B).
Question 4: The number of aldol products possible when acetone reacts with acetaldehyde is:
A. 1
B. 2
C. 3
D. 4
Solution:
Possible condensations:
- Self-aldol of acetone
- Self-aldol of acetaldehyde
- Cross-aldol between acetone and acetaldehyde
Hence, 3 products are possible.
Hence, the correct answer is option (C).
Frequently Asked Questions (FAQs)
No, condensation and precipitation are not the same. Condensation refers to the process of water vapor turning into liquid, while precipitation involves water falling from the atmosphere to the earth in forms like rain, snow, sleet, or hail.
Condensation is primarily caused by the cooling of air. When warm air rises and meets cooler surfaces or air, its temperature drops, and the moisture within it condenses into liquid. Common occurrences include dew forming on grass in the morning and water droplets on a cold glass.
Condensation occurs in various places, including:
- On the outside of cold beverage containers
- On windows during cold weather
- In bathrooms after hot showers
- On mirrors
- In kitchen areas when boiling water is present
The different forms of condensation are:
Fog
Cloud
Mist
Dew
Condensation is the water vapour process that turns back into liquid water, with these huge fluffy clouds floating over your head as the best example. When the water droplets come together in clouds, they get heavy enough to rain on your head.
A peptide bond is created in combination with the amino group of the other molecule by the carboxyl groupset which releases a water molecule (H2O). This is a condensation process between the amino acids (also known as a dehydration process).
The condensation reaction examples are listed below:
Acyloin condensation
Aldol condensation
Esterification
Doebner–Miller reaction
Questions related to
On Question asked by student community
Correct Answer: Nylon 66
Solution : The correct option is Nylon 66.
The polymer formed by the reaction of hexamethylene diamine with adipic acid is known as "Nylon 66". It is a synthetic polyamide made up of repeating units produced from these two monomers. Nylon 66 is well-known for
Correct Answer: Terephthalic acid
Solution : The correct answer is Terephthalic acid.
Ethylene glycol and terephthalic acid are combined in a condensation reaction to create terylene, often known as polyester. Water molecules are eliminated during this reaction as the two monomers join together to form a polymer chain.
Correct Answer: burning Deuterium in oxygen
Solution : The correct option is the burning of deuterium in oxygen.
Heavy water, or deuterium oxide (D2O), is produced by burning deuterium in oxygen. Burning deuterium (a heavy isotope of hydrogen) in oxygen-containing air can result in the production of
