Coordination Number - Overview, Definition, Factor, Examples, FAQs
Have you ever wondered why some metal ions in a compound can bind to more atoms or ions than others? What determines the number of atoms, ions, or molecules that can surround a central metal ion in a complex? The answer is a coordination compound. The total number of atoms, ions, and molecules bonded to the central metal atom in a coordination complex is called the coordination number. Another term used for the coordination number is ligancy, which refers to the number of atoms bonded to the central metal atom, and is generally referred to as ligands.
This Story also Contains
- Coordination Number
- Factors Affecting Coordination Number
- How to Find the Coordination Number of the Central Atom:
- Coordination Number Examples
- Geometry of Molecules Based on Coordination Number
- Some Solved Examples
In this article, we cover the concept of coordination numbers, which fall under the Coordination Compounds. It is an important topic of class 12 for board exams and also for the JEE Main exam and the National Eligibility Entrance Test (NEET).
Coordination Number
Do you know how to define the Coordination number? It is defined as the total number of atoms, ions, or molecules attached to an atom in a specific molecule or crystal and is referred to as the coordination number of that atom. The coordination number of an atom is often referred to as its ligancy.
The ligands are the atoms, ions, or molecules that are attached to the center atom (or molecule/ion). When computing the coordination number of a central atom in a crystal, the legacy of molecules is computed differently.
According to the radius ratio, “The larger the charge, the smaller the ion becomes, limiting the number of groups that can coordinate.”
Factors Affecting Coordination Number
Different types of forces hold the atoms together in these complexes and contribute to the observed coordination numbers. In fluoride complexes, the bonds to the extremely electronegative fluorine atoms are virtually ionic. Therefore increase in coordination number with fluoride ions from 4 to 6 to 7 for $\mathrm{B}^{3+}, \mathrm{Fe}^{3+}$, and $\mathrm{Zr}^{4+}$ is primarily due to the cation's increased size. This allows a growing number of fluoride ions to be packed around the center ion.
1. Size of the Central Metal Ion
-
Larger metal ions can accommodate more ligands around them.
-
Smaller metal ions generally have lower coordination numbers.
Example:
$\begin{aligned} & {\left[\mathrm{AlF}_6\right]^{3-} \rightarrow \text { C.N. }=6} \\ & {\left[\mathrm{BeF}_4\right]^{2-} \rightarrow \text { C.N. }=4}\end{aligned}$
2. Size of the Ligands
-
Small ligands can pack closely, allowing a higher coordination number.
-
Bulky ligands cause steric hindrance, reducing C.N.
Example:
- $\left[\mathrm{FeF}_6\right]^{3-} \rightarrow$ C.N. $=6$
- $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-} \rightarrow$ C.N. $=6$
- $\left[\mathrm{Fe}\left(\mathrm{PPh}_3\right)_4\right] \rightarrow$ C.N. $=4$
3. Charge on the Central Metal Ion
-
Higher positive charge increases attraction towards ligands, favoring a higher coordination number.
Example:
- $\mathrm{Fe}^{3+} \rightarrow$ usually C.N. $=6$
- $\mathrm{Fe}^{2+} \rightarrow$ may show lower C.N. in some complexes
4. Nature of the Ligand (Denticity)
- Monodentate ligands occupy one coordination site.
- Polydentate ligands occupy multiple sites, influencing C.N.
Example:
- $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+} \rightarrow$ C.N. $=6$
- $\left[\mathrm{Co}(\mathrm{en})_3\right]^{3+} \rightarrow \mathrm{C} . \mathrm{N} .=6$ (en is bidentate)
5. Electronic Configuration of the Metal Ion
- Certain electronic arrangements are more stable with specific coordination numbers.
- Transition metals often prefer C.N. = 6 or 4.
Example:
- $d^8$ metals like $\mathrm{Ni}^{2+}, \mathrm{Pd}^{2+}, \mathrm{Pt}^{2+}$ often form square planar (C.N. $=4$ ) complexes.
7. Steric Hindrance
-
Increased crowding around the metal center lowers coordination number.
Example:
$\left[\mathrm{Ni}(\mathrm{CO})_4\right] \rightarrow$ C.N. $=4$ (bulky CO ligands)
Also, check-
How to Find the Coordination Number of the Central Atom:
The coordination number related to a given atom in polyatomic ions and molecules can be computed by counting the total number of atoms it is bound. Whether it is a single bond or a double/triple bond, all are included to calculate the coordination number. Using the polyatomic ion [Cr(NH3)2Cl2Br2]– as an example, the coordination number of the core cation (Cr3+) can be calculated by counting the total number of atoms linked to the chromium atom, which is 6. This means the coordination number is 6.
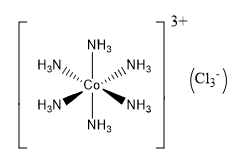
Because the center cobalt atom is connected to six different nitrogen atoms in the example above, the coordination number of the central cobalt atom is six. The bonds between crystals are less obvious in their solid-state forms. In such cases, the coordination number of the center atom reflects the number of arrangements of neutrons around the atom in question. The total number of atoms that surround a specific atom in a crystal is determined by the atom's position in the crystal. In the case of crystals, there are two separate metrics of ligancy- the bulk coordination number and the surface coordination number. Also, students can find related topics below.
Also Check-
Coordination Number Examples
A coordination number of a crystalline solid is the number of atoms, ions, or molecules that a central atom/ion has as its nearest neighbors. For example, the coordination numbers of Pt and Fe in the complex ions $\left[\mathrm{PtCl}_6\right]^{2-}$ and $\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ are 6 and 6, respectively. Pt and Fe are linked to six ligands, Cl and H2O, respectively.
$\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2 \mathrm{Br}_2\right]^{-}$ is another example. Because the total number of atoms/ions/molecules linked to Cr is discovered to be 6, the core atom Cr has a coordination number of 6. The bidentate ligand, the coordination number Co is 6 in the complex ion [Co(en)3 ]3+. Below, students can find coordination numbers with some examples.
-
$\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)_2\right]^{+}$, where Ag has a coordination number of 2 and the compound's molecular shape is linear.
-
$\left[\mathrm{NiCl}_4\right]_2$, where Ni has a coordination number of 4 and the compound's molecular shape is square planar.
-
$\left[\mathrm{CoCl}_6\right]_3$ is a chemical with the coordination number 6 and an octahedral molecular shape.
-
The molecular geometry of$\left[\mathrm{ZrF}_7\right]_3$ is a pentagonal bipyramid, with Zr having a coordination number of 7.
-
$\left[\mathrm{CoCl}_5\right]_2$ is a chemical with the coordination number 5 and a trigonal bipyramidal molecular shape.
Geometry of Molecules Based on Coordination Number
We can calculate the geometry of a molecule using the coordination number. A list is given below with the corresponding geometry and coordination number.
|
Coordination number |
Molecular Geometry |
|
2 |
Linear |
|
3 |
Trigonal Planar |
|
3 |
T-shaped |
|
3 |
Trigonal Pyramidal |
|
4 |
Tetrahedral |
|
4 |
Square Planar |
|
5 |
Trigonal Bipyramidal |
|
5 |
Square Pyramid |
|
6 |
Octahedral |
|
7 |
Pentagonal Bipyramidal |
|
7 |
Capped Octahedron |
|
8 |
Square Antiprism |
|
8 |
Dodecahedron |
|
8 |
Hexagonal Bipyramidal |
|
9 and above |
Other complex structures |
There are different types of lattices, like BCC, FCC, and many more. The abbreviation for BCC is body-centered cubic, and the coordination number of the bcc atom is 8. The abbreviation of FCC is face-centered cell, and the coordination number is 12. The abbreviation of CCP stands for cubic close-packed, and the coordination number is 12. Similarly, hcp stands for hexagonal close-packed cell, and the coordination number of hcp is 12. The coordination number of the simple cubic seems to have a coordination number of 6, and each unit cell contains one atom.
NCERT Chemistry Notes :
Some Solved Examples
Question 1: The coordination number of a central metal atom in a complex is determined by
1) (correct)the number of ligands around a metal ion bonded by sigma bonds
2)the number of ligands around a metal ion bonded by pi-bonds
3)the number of ligands around a metal ion bonded by sigma and pi-bonds, both
4)the number of only anionic ligands bonded to the metal ion.
Solution:
The coordination number is the number of ligands that are bonded directly to the metal by coordinate bonds. Thus, the coordination number is the number of ligands around a metal atom/ion bonded by sigma bonds.
Hence, the answer is option (1).
Question 2: The coordination number of HCPs is
1) 8
2) 10
3) 6
4) (correct)12
Solution:
As we learned in
Coordination number -
The total number of atoms touching a particular atom in the given unit cell is known as the coordination number, and those atoms are known as the nearest neighbors.
wherein
Unit cell Coordination number
- Primitive 6
- BCC 8
- FCC 12
- HCP 12
In HCP packing, the coordination number of atoms is 12.6 in the Same plane, 3 in the upper plane, and 3 in the lower plane.
Hence, the answer is option (4).
Question 3: The coordination number of copper in Cuprammonium sulfate is
1) 2
2) 6
3) (correct)4
4) 4
Solution:
In Cuprammonium sulfate$\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right] \mathrm{SO}_4$, there are four ammonia ligands bonded to the central metal ion through coordinate bonds.
Thus, the coordination number of Cu is 4.
Hence, the answer is option (3).
Question 4: The Yellow compound of lead chromate gets dissolved on treatment with a hot NaOH solution. The product of lead formed is a :
1) Tetraanionic complex with coordination number six
2) Dianionic complex with coordination number six
3) Neutral complex with coordination number four
4) (correct) Dianionic complex with coordination number four
Solution: $\mathrm{PbCrO}_4+\mathrm{NaOH} \longrightarrow \mathrm{Na}_2\left[\mathrm{PbO}_2\right]^{2-}+\underset{\text { Dianimic Eq. C.N. }=4}{\mathrm{Na}_2\left[\mathrm{CrO}_4\right]^{2-}}$
Hence, the answer is option(4).
Practice More Questions With The Link Given Below:
Also, Refer To
Frequently Asked Questions (FAQs)
Ligands are ions or molecules that donate electron pairs to the central metal ion, forming coordinate bonds. The properties of ligands, such as their size and charge, significantly influence the coordination number, as well as the overall stability and geometry of the complex.
When a central metal atom is surrounded by 12 ligands or atoms and ions . its coordination number is 12.
There are multiple factors that affect the coordination number like the charge which depends on the electromagnetic configuration of the metal ion, the size of the metal ion, and many more.
Common coordination numbers include 2, 4, 6, 8, and occasionally 12. A coordination number of 6 is typical for octahedral complexes, 4 for tetrahedral complexes, and 2 for linear complexes.
There are some examples of coordination number
[Ag(NH3)2]+, where Ag has a coordination number of 2 and the compound's molecular shape is linear.
[NiCl4]2, where Ni has a coordination number of 4 and the compound's molecular shape is square planar.
[CoCl6]3 is a chemical with the coordination number 6 and an octahedral molecular shape.
The coordination number means a number of atoms surrounded by a central atom. Coordination number of 5 means the central ion is surrounded by 5 molecules or legends.
The coordination number can be determined by examining the molecular structure of a coordination complex. It is the total count of the atoms or ions directly bonded to the central metal.
the coordination number depends on the structure of the metal as the larger the size of metal more the legends. as well as it depends on the charge on the metal.
A unit cell is the smallest repeating unit that has full symmetry of the crystal structure and it is defined as a parallelepiped, with the six lattice parameters taken as the lengths of the cell edges (a, b, c) and the angles between them (α, β, γ).
A coordination number is a number of legends bounded by central metal. For example, in the given compound [Cr(NH3)2Cl2Br2]−, Cr3+ is a central atom that has six legends bounded with it therefore the coordination number is 6 and the compound is described as a hexacoordinate.
