Empirical and Molecular Formula: Definition, Questions and Examples
We know that the formula of water is $\mathrm{H}_2 \mathrm{O}$ while that of sulphuric acid is $\mathrm{H}_2 \mathrm{SO}_4$. Similarly, the formula of carbon dioxide is CO2, while that of ammonia is NH3. Thus a formula is a symbolic representation of one molecule of the substance which tells the number and kinds of atoms of various elements present in its molecule.
This Story also Contains
- What is a Chemical Formula?
- Empirical Formula
- Molecular Formula
- Some Solved Example
- Practice more Questions from the link given below:
- Summary
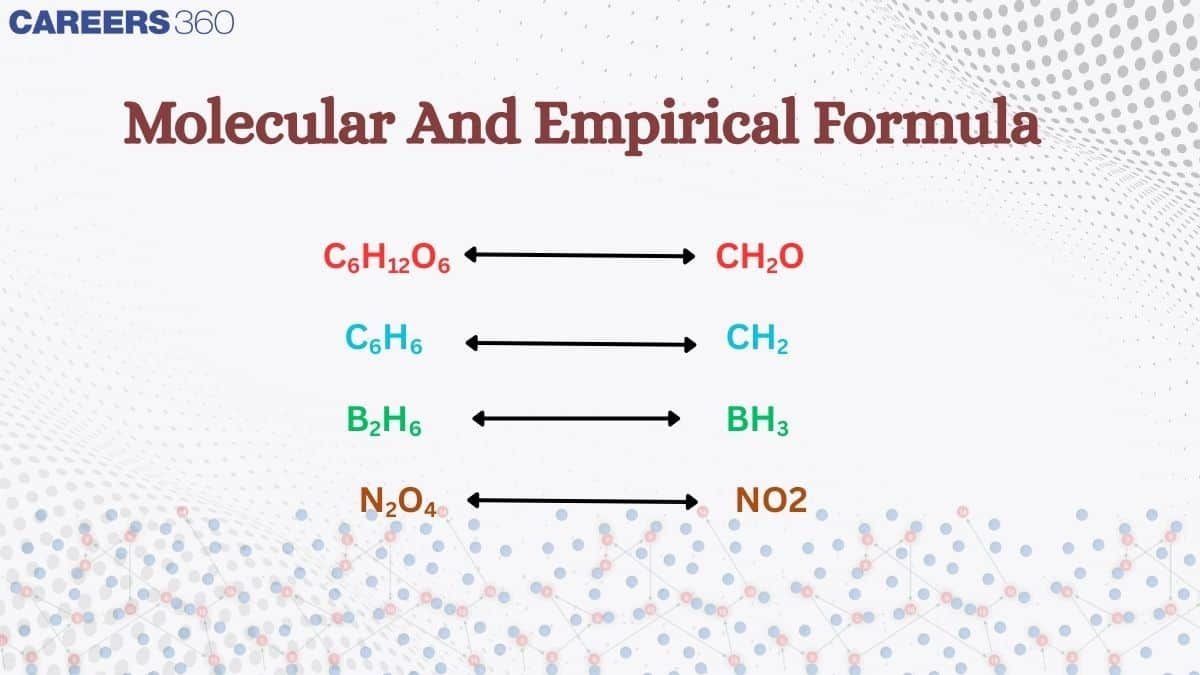
The formula which gives the simplest whole number ratio of the atoms of various elements present in one molecule of the compound is called the empirical formula whereas the formula which gives the actual number of atoms of various elements present in one molecule of the compound is called molecular formula.
What is a Chemical Formula?
A chemical formula represents the combination of atoms of all the elements that make up a compound. It represents the relative ratio of atoms of its constituent elements. In the case of a compound, it represents one molecule, one mole, and one gram molecular weight of the compound.
For example, $\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}$ represents one molecule, one mole, and one gram molecular weight of hydrated copper sulfate.
Empirical Formula
It is the simplest ratio of the number of atoms of different elements present in one molecule of a compound. It does not represent the actual number of atoms of different elements present in one molecule of the compound.
How to find out the empirical formula and the molecular formula in case the % composition of the compound is given to us
Step 1. Conversion of a mass percent to grams.
Step 2. Convert grams into the number of moles of each element.
Step 3. Divide the mole value obtained above by the smallest number.
Step 4. Write the empirical formula by mentioning the numbers obtained above after writing the symbols of respective elements.
Step 5. Write molecular formula with the help of the information given.
Molecular formula is a whole number multiple of the empirical formula
Molecular formula $=(\text { Empirical formula })_{\mathrm{n}}$
where n is the whole number.
Molecular Formula
The molecular formula of a compound is the chemical formula that represents the true formula of its molecule. It expresses the actual number of atoms of various elements present in one molecule of the compound.
-
n = (Molecular weight) / (Empirical formula weight)
-
Molecular weight can be directly given or some other information like Vapour density can be given which will enable us to calculate the molecular weight.
-
Molecular weight = 2 x Vapour Density
-
For some compounds, the molecular formula and empirical formula may be the same also.
Also Read:
Recommended topic video on(Empirical and Molecular Formula )
Some Solved Example
Que 1. A 5.325 g sample of Methyl Benzoate, a compound used in the manufacturing of perfumes, is found to contain 3.758g of Carbon, 0.316g of Hydrogen and 1.251g of Oxygen. What is the empirical formula of the compound?
1) C4H4O
2) C2H4O
3) C2H2O
4) C4H3O
Solution
For glucose, the empirical formula is CH2O
|
Element |
% |
Mole ratio |
Simplest mole ratio |
|
C | $\frac{3.758 \times 100}{5.325}=70.57$ | $\frac{70,57}{12}=5.88$ | $\frac{5.88}{1.47}=4$ |
|
H | $\frac{0.316 \times 100}{5.325}=5.93$ | $\frac{5.93}{1}=5.93$ | $\frac{5.93}{1.47}=4$ |
|
O | $\frac{1.251 \times 100}{5.325}=23.50$ | $\frac{23.50}{16}=1.47$ | $\frac{1.47}{1.47}=1$ |
So, the Empirical formula is C4H4O
Hence, the answer is an option (1).
Que 2: An organic compound containing C & H has 92.3% C. Its empirical formula is:
1) CH2
2) CH3
3) CH4
4) CH
Solution
|
Element |
% |
Atomic Mass |
Relative no. of atoms |
Simplest ratio |
|
C |
92.30 |
12 |
7.69 |
1 |
|
H |
7.70 |
1 |
7.70 |
1 |
So, its empirical formula is CH
Hence the answer is an option (4).
Que 3. A compound of Carbon, Hydrogen and Nitrogen contains these elements in the ratio 9:1:3.5. Calculate the empirical formula.
1) C3H8N
2) C3H4N
3) C2H4N
4) C3H2N
Solution
| Element | Element ratio | Atomic Mass | Relative no. of atoms | Simplest ratio |
| C | 9 | 12 | $\frac{9}{12}=0.75$ | $\frac{0.75}{0.25}=3$ |
| H | 1 | 1 | $\frac{1}{1}=1$ | $\frac{1}{0.25}=4$ |
| N | 3.5 | 14 | $\frac{3.5}{14}=0.25$ | $\frac{0.25}{0.25}=1$ |
So Empirical formula is C3H4N.
Hence, the answer is an option (2).
Que 4. A compound has an empirical formula C2H4O. An independent analysis gave a value of 132 for its molecular mass. What is the correct molecular formula?
1) C4H4O5
2) C10H12
3) C6H12O3
4) C4H8O5
Solution
$\mathrm{n}=\frac{\text { Molecular Mass }}{\text { Empirical Formula mass }}=\frac{132}{44}=3$
Therefore, Molecular formula is (C2H4O)3 = (C6H12O3)
Hence, the answer is an option (3).
Que 5. An organic compound contains 49.3% C, 6.84% H and the remaining is oxygen and its vapor density is 73. The molecular formula of the compound is
1) C3H8O2
2) C6H9O
3) C4H10O2
4) C6H10O4
Solution:
Molecular mass $=2 \times 73=146$
% of oxygen = 100 - (49.3 + 6.84)
= 43.86%
Thus we have,
$\begin{aligned} & C=\frac{\%}{100} \times \frac{\text { Molecular mass }}{\text { Atomic mass }}=\frac{49.3}{100} \times \frac{146}{12}=6 \\ & H=\frac{\%}{100} \times \frac{\text { Molecular mass }}{\text { Atomic mass }}=\frac{6.84}{100} \times \frac{146}{1}=10 \\ & O=\frac{\%}{100} \times \frac{\text { Molecular mass }}{\text { Atomic mass }}=\frac{43.86}{100} \times \frac{146}{16}=4\end{aligned}$
Thus, Molecular Formula = C6H10O4
Hence, the answer is an option (4).
Que 6. A compound on analysis was found to have the following composition: (i) Sodium 14.31%, (ii) Sulphur 9.97%, (iii) Oxygen 69.5%, (iv) Hydrogen 6.22%. Calculate the molecular formula of the compound assuming that the whole of Hydrogen in the compound is present as water of Crystallization. The molecular mass of the compound is 322.
1) Na2SH20O10
2) Na2SH20O14
3) Na2SH20O7
4) Na2SH30O16
Solution
|
Element |
%age |
Atomic mass |
Relative no of atoms |
Simplest Ratio |
|
Na |
14.31 |
23 |
0.622 | $\frac{0.622}{0.311}=2$ |
|
S |
9.97 |
32 |
0.311 | $\frac{0.311}{0.311}=1$ |
|
H |
6.22 |
1 |
6.22 | $\frac{\mid 6.22}{0.311}=20$ |
|
O |
69.50 |
16 |
4.34 | $\frac{4.34}{0.311}=14$ |
Empirical formula = Na2SH20O14
Empirical formula mass
$$
=2 \times 23+32+20 \times 1+14 \times 16=322
$$
Molecular mass = 322
Molecular Formula = $\mathrm{Na}_2 \mathrm{SH}_{20} \mathrm{O}_{14}$
Hence, the answer is an option (2).
Practice more Questions from the link given below:
Summary
In a chemical formula, the actual number of atoms are represented in the compound. Chemical formula shows the exact number of atoms present in it and their composition and the ratio of each element in a molecule. Whereas the empirical formula of a compound is the chemical formula which expresses the simplest whole number ratio of the atoms of the various elements present in one molecule of the compound.It show the number of atoms but not in exact quantity in the relative proportion.
