Oleum and its % labelling
Oleum referred to as fuming sulfuric acid, is a concentrated solution of sulfur trioxide, $\mathrm{SO}_3$, in sulfuric acid, H2SO4. The chemical compound retains a very key position in various industrial processes because of its strongly acidic properties and application as a dehydrating agent. Oleum is formed upon dissolving SO₃ in concentrated sulfuric acid, which results in a viscous liquid. Actually, it is this free sulfur trioxide that really presents what makes oleum most interesting and useful in chemical synthesis.
This Story also Contains
- Classifications and Types of Oleum
- Oleum and its % Labeling
- Industrial Applications and Relevance in Real Life
- Some Solved Examples
- Practice More Questions From the Link Given Below:
- Summary
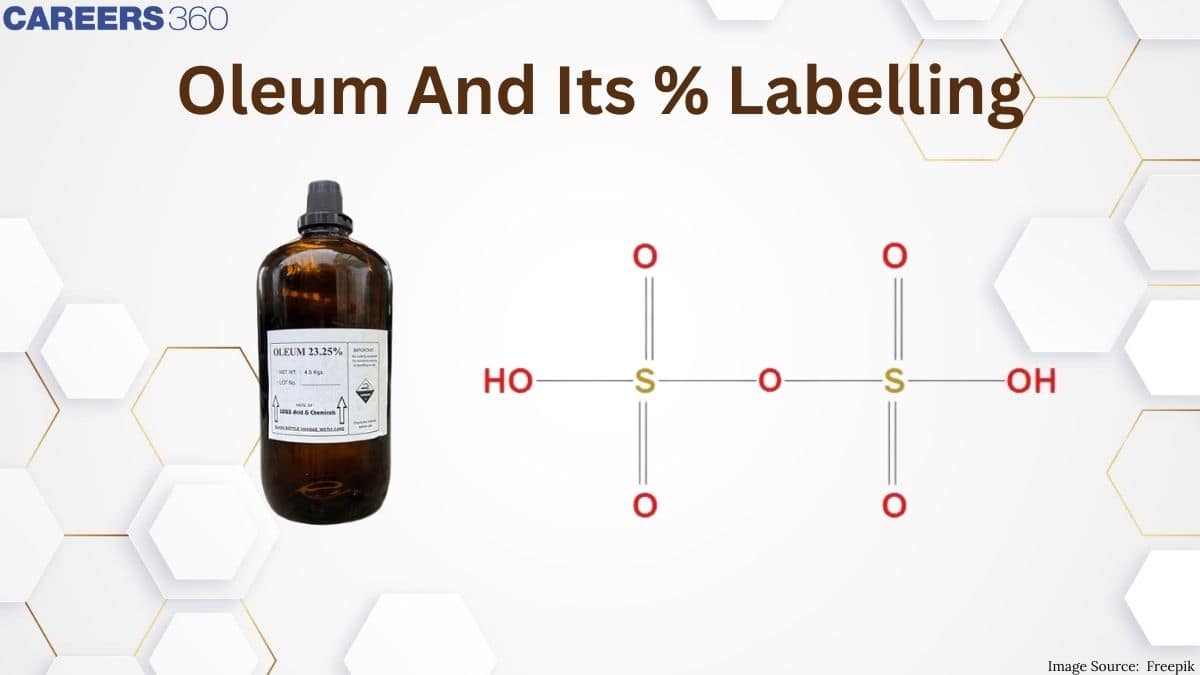
One of the most critical aspects used in labeling oleum is the percentage, related to the total mass of H₂SO₄ obtainable when a certain amount of oleum is diluted with water. For example, if an oleum sample was labeled as 109% H2SO4 then the addition of 9 grams of water to 100 grams of oleum is enough to result in 109 grams of pure H2SO4 This percentage labeling is very important to the chemist and industries that need specific concentrations for various reactions and application in such industries. The understanding of the implications of these percentages becomes quite important in ensuring safe handling of oleum and its effective use in industrial settings.
Classifications and Types of Oleum
Oleum can be classified based on the percentage label it carries that describes the amount of free $\mathrm{SO}_3$ available in the solution. The percentage labeling is usually supra-100%, which describes the concentration of H2SO4 that a certain oleum will produce upon dilution. For instance, in 104%H2SO4 oleum, there will be 4 grams of free $\mathrm{SO}_3$ in 100 grams of oleum while 118% oleum will contain 18 grams of free SO₃. The free SO₃ is important because it defines the reactivity and acidity of the oleum. The greater the percentage, the more water is required to convert free $\mathrm{SO}_3$ into H2SO4 for industrial use.
Oleum and its % Labeling
Oleum is a mixture of $\mathrm{SO}_3$ dissolved in $100 \% \mathrm{H}_2 \mathrm{SO}_4$. The strength of the Oleum sample is expressed in terms of % labeling and it is defined as the grams of pure H2SO4 that can be obtained from 100 g of the Oleum sample upon dilution with water.
For example, if an Oleum sample is labeled as 109%, it means that upon the addition of 9g water to 100 g of Oleum sample, the amount of H2SO4 obtained is 109 g.
We can calculate the % of free SO3 in the sample by simple stoichiometry as follows:
$\mathrm{SO}_3+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_4$
Weight of H2O added = 9 g
Moles of H2O added = 0.5
Moles of $\mathrm{SO}_3$ present = 0.5 (from reaction stoichiometry)
Weight of $\mathrm{SO}_3$ in the 100g Oleum sample = $0.5 \times 80$= 40 g
% of free $\mathrm{SO}_3$ in Oleum = 40 %
Let us also discuss a general case:
Let us suppose that the % labeling of the Oleum sample is $y \%$. This means that $(\mathrm{y}-100) \mathrm{g}$ of water is added to 100 g Oleum sample
$
\begin{gathered}
\text { moles of water }=\frac{y-100}{18}=\text { moles of } \mathrm{SO}_3 \\
\text { of free } \mathrm{SO}_3 \text { in Oleum }=\frac{80 \times(y-100)}{18}
\end{gathered}
$
In this manner, the % of free $\mathrm{SO}_3$ in the Oleum sample can be calculated.
Industrial Applications and Relevance in Real Life
Oleum is crucial in modern industry, especially in the production of sulfuric acid—one of the most widely used chemicals globally. As a concentrated solution of sulfur trioxide in sulfuric acid, oleum enables precise control over acid strength by allowing titration of free $\mathrm{SO}_3$ content, or effectively supplying “over‑100%” sulfuric acid.
In chemical education, oleum offers precious classroom applications. Its role in acid–base titrations, stoichiometric calculations, and demonstrations of industrial chemistry principles provides a absorbing case study on the importance of precise measurement, concentration control, and understanding exothermic reactions in practical laboratory or industrial scenarios.
Also Read:
Some Solved Examples
Example 1
A sample of oleum is labeled as 104.5%. What is the percentage of free $\mathrm{SO}_3$ in the sample?
1)20%
2)40%
3)60%
4)80%
Solution:
$\mathrm{SO}_3+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_4$
Weight of H2O added =4.5 g
Moles of H2O added = 0.25
therefore Moles of SO3 present = 0.25
therefore Weight of SO3 in the 100g Oleum sample = 0.25 times 80 = 20 g
therefore % of free SO3 in Oleum = 20 %
Example 2
An Oleum sample contains 25% w/w of free $\mathrm{SO}_3$. What is its % labelling?
1)105.625
2)107.25
3)114.525
4)122.5
Solution:
$\mathrm{SO}_3+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_4$
% of free SO3 in Oleum = 25 %
therefore Weight of SO3 in the 100g Oleum sample = 25 g
therefore Moles of SO3 present = 25/80 = 0.3125
therefore Moles of H2O added = 0.3125 X 18
therefore Weight of H2O added = 5.625 g
therefore % labelling of Oleum= (100+ 5.625) = 105.625 %
Example 3
The % of free SO3 in an Oleum sample is 20%. What is its labelling?
1) 104.5
2) 105.6
3) 107.8
4) 109.1
Solution:
$\mathrm{SO}_3+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_4$
% of free SO3 in Oleum =20 %
therefore Weight of SO3 in the 100g Oleum sample = 20 g
therefore Moles of SO3 present = 20/80 = 0.25
therefore Moles of H2O added = 0.25 X 18
therefore Weight of H2O added = 4.5 g
therefore % labelling of Oleum= (100+ 4.5) = 104.5 %
Example 4
What is the % of free SO3 in oleum, that is labeled 118%?
1) 20%
2) 40%
3) 60%
4) 80%
Solution:
$\mathrm{SO}_3+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_4$
Weight of H2O added = 18 g
Moles of H2O added = 1
therefore Moles of SO3 present = 1
therefore Weight of SO3in the 100g Oleum sample = 1 times 80 = 80 g
therefore % of free SO3 in Oleum = 80 %
Practice More Questions From the Link Given Below:
Summary
In the final sense, oleum is a solution of sulfur trioxide that is highly concentrated in sulfuric acid, characterized by its diverse unique properties and huge industrial applications. The percentage labeling of oleum is therefore important, as it represents the concentration of H₂SO₄ to be obtained after dilution; it is therefore very important for chemists and industries handling this substance. Close control over acidity and stoichiometry ensures proper reaction conditions, safe handling, and consistency in industrial and laboratory processes.
