Some Basic Concept in Chemistry Formula: Definition, Questions, Examples
Many consider chemistry to be a very complicated course filled with complex theories and formulae yet are deeply sought after. The truth is, it tries to describe the basic nature of elements and what the reactions are happening between substances in reality. This article presents a number of basics in chemistry, talking about the definitions, types, and applications that do exist in real life. It is an elaborately diversified subject, dealing with the study of matter, its composition, and changes.
This Story also Contains
- Outline of Basic Chemistry Concepts
- Importance and Scope of Chemistry
- Some Solved Examples
- Practice More Questions From the Link Given Below:
- Conclusion

Outline of Basic Chemistry Concepts
The basics of chemistry deal with some principles according to which matter is governed. Some of the important definitions includes that of matter, which states any substance possessing mass and occupying space refers to matter, and element, which is a pure substance comprising atoms of only one kind. Also, compounds result from a combination of two or more elements in fixed ratios, such as water (H₂O) or even sodium chloride (NaCl).
Another core definition is that of mole, it may be described as a unit used to measure the magnitude of the substance; one mole is 6.022×1023 entities. It tells us the number of particles in a mole is equal to the constant referred to as Avogadro's number, and it provides a practical application in the real world. Another mandate of chemistry is the law of mass conservation, which states that mass is neither created nor destroyed in a chemical reaction. Consequently, chemical equations must be balanced. From the above simple concepts, more sophisticated studies in chemistry can be created.
Heating of Carbonates
- Group II metal carbonates like MgCO3, CaCO3 and other bivalent metal carbonates (PbCO3, ZnCO3) liberate CO2 on heating and leave their oxide as residue
$\mathrm{MCO}_3 \xrightarrow{\Delta} \mathrm{MO}+\mathrm{CO}_2$
- Group 1 metal carbonates like Na2CO3, K2CO3 are resistant to decomposition upon heating (except Li)
Heating of Bicarbonates
- Group II metal bicarbonates like Ca(HCO3)2 liberate CO2 and H2O on heating and leave their oxide as residue
$\mathrm{M}\left(\mathrm{HCO}_3\right)_2 \xrightarrow{\Delta} \mathrm{MO}+2 \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
- Group I metal bicarbonates like NaHCO3 liberate CO2 and H2O on heating and leave their carbonate as residue
$2 \mathrm{Na}\left(\mathrm{HCO}_3\right) \xrightarrow{\Delta} \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
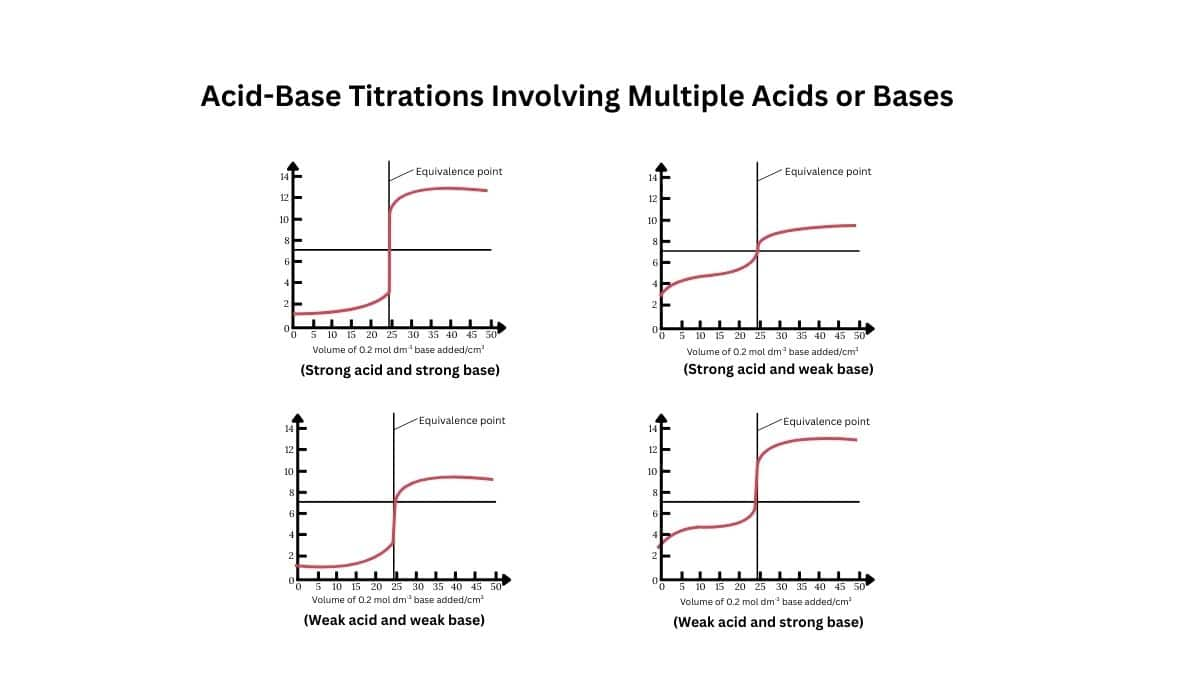
Acid-Base Titrations Involving Multiple Acids or Bases
Acid–base titrations involving multiple acidic or basic components—like polyprotic acids—proceed in various stages. Each ionizable proton is neutralized separately, generating individual equivalence points on the titration curve. For a diprotic acid there are two such points, for a triprotic acid, up to three. Well, some may merge if dissociation constants are similar. Between these points, buffer regions form where the pH changes gradually, often aligning with pKa values at half‑equivalence stages.
Importance and Scope of Chemistry
We know that chemistry deals with the study of composition, structure and properties of matter and the changes which the matter undergoes under different conditions and the laws which govern these changes.
Chemistry deals with:
1.) Increase in the production of food through use of chemical fertilizers, insecticides, fungicides and pesticides.
2.) Contribution to better health and sanitation through the use of life saving drugs, e.g., cisplatin and taxol for cancer and AZT for AIDS and also through the use of analgesics, antibiotics, tranquilizers, antiseptics, disinfectants, insecticides and anaesthetics.
3.) Saving the environment by use of environment friendly chemicals.
4.) Increase in comfort and luxury by use of synthetic fibres, building materials, metals like iron, aluminium, copper, silver, gold etc. and articles of domestic use like soaps, detergents, paper, glass, plastics etc.
5.) Nuclear and atomic energy involving use of uranium and plutonium for nuclear reactors.
6.) Transport which involves use of petrol and diesel and other high quality fuels for aeroplanes etc.
Also Read:
Some Solved Examples
Example 1
Question: A solid mixture (10g) consisting of operator name MgCO3 and Na2CO3 was heated until the mass of the residue was constant. If the loss in mass is 44%, find the amount of MgCO3 and Na2CO3 in the mixture respectively.
1) 8.4g, 1.6g
2) 1.6g, 8.4g
3) 5g, 5g
4) 8.8g, 1.2g
Solution
The reactions occur as follows:
$\mathrm{MgCO}_3 \xrightarrow{\Delta} \mathrm{MgO}+\mathrm{CO}_2$
$\mathrm{Na}_2 \mathrm{CO}_3 \xrightarrow{\Delta} x$
Mass is lost in the form of CO2
Thus, the weight of CO2 = 4.4g
Thus, moles of CO2 = 0.1 = moles of MgCO3
Therefore, mass of MgCO3 in the mixture = 8.4g
Thus, mass of Na2CO3 in the mixture = 1.6g
Hence, the answer is the option (1).
Example 2
Question: A 20 g mixture of Na2CO3 and CaCO3 is gently heated and produces 2.24 liters of CO2 at STP. What is the % weight of Na2CO3 in that sample?
1) 12.5%
2) 25%
3) 50%
4) 75%
Solution
We know that Na2CO3 does not decompose on heating. So CO2 will be produced by only CaCO3CaCO3(s)→CaO(s) + CO2(g)By reaction stoichiometry
Mole of CaCO3 = mole of CO2
Mole of CaCO3 = 2.24 litre
A mole of CaCO3 = 2.24/22.4 mole
A mole of CaCO3 = 0.1 moles Now,
Weight of CaCO3 = mole X molar mass of CaCO3
Weight of CaCO3 = 0.1 X 100 = 10g
wt. of Na2CO3 = 20g - wt. of CaCO3
wt. of Na2CO3 = 20g - 10 = 10g
% wt. of Na2CO3 = (10/20)X100 % = 50%
Hence, the answer is the option (3).
Example 3
Question: 25 ml of a solution containing HCl and H2SO4 requires 25 ml of 0.5M caustic soda for complete neutralization. 50 ml of the same solution on precipitation with excess BaCl2 gave 2.33 g of precipitate BaSO4. What is the strength of HCl in the solution (in g/L)?
1)36.5
2) 3.65
3)7.3
4)73
Solution
Let the molarity of HCl and H2SO4 be x and y respectively.
Now, upon neutralization, we have:
25(x+2y) = 25 x 0.5
x + 2y = 0.5
Next, upon precipitation, we have:
50 x y = (2.33/233) x 1000 = 10
y = 0.2 and x = 0.1
Thus, the molarity of HCl = 0.1
It means 3.65 g/L of HCl is present in the stock solution.
Hence, the answer is the option (2).
Practice More Questions From the Link Given Below:
Conclusion
Basics of chemistry therefore form the basis by which one would grasp issues about matters and their interaction. The definition of matter, elements, compounds, and moles are among the basic things a student should know as a professional. It is the identification of several forms of mixtures and chemical reactions that bring out the true complexity of the chemical process. The above concepts have applications in real life, from cookery and health to offering exploration in environmental science and industry.
