Percent Composition Formula: Definition, Questions and Examples
To determine the molecular formula of a compound, we begin by measuring the mass or mass percentage of each element present in the compound. The composition is generally expressed as the mass percentage composition. The mass percentage gives the mass of each element expressed as the percentage of the total mass. In other words, it gives the number of grams of the element present in 100 g of the compound.
This Story also Contains
- Percentage Composition:
- Equivalent Weight:
- Some Solved Examples
- Summary
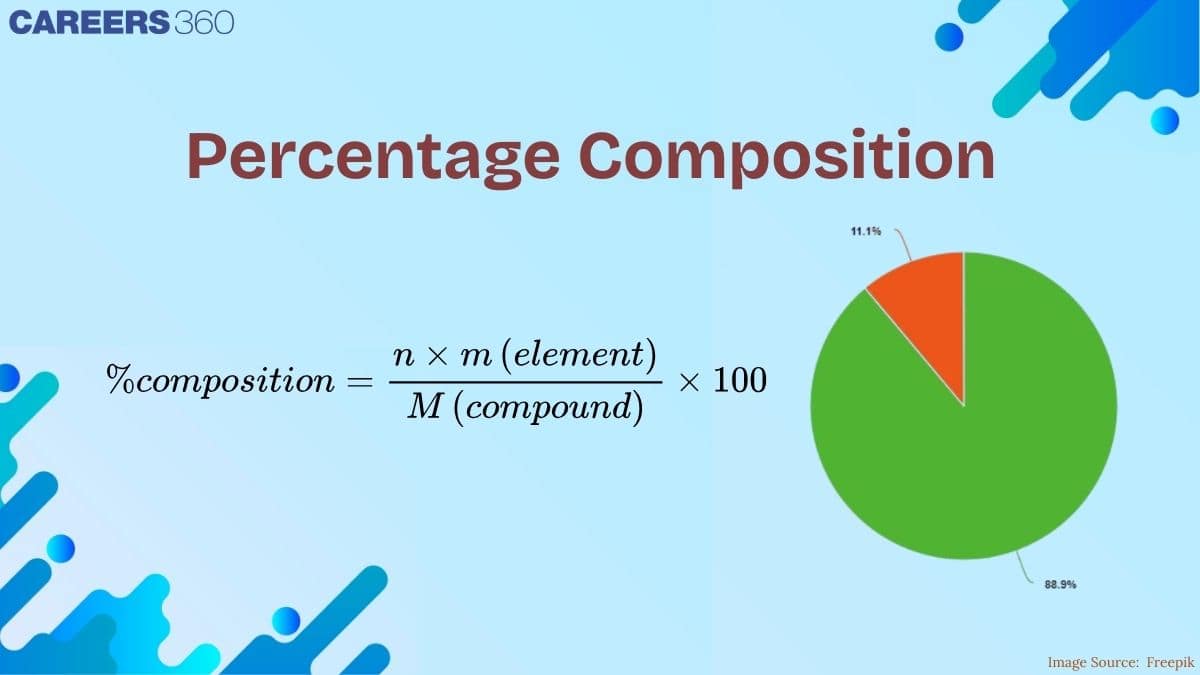
Percentage Composition:
The percentage of any element or constituent in a compound is the number of parts by mass of that element or constituent present in 100 parts by mass of the compound.
Mass
Let us take an example of water (H2O), it contains hydrogen and oxygen, and the percentage composition of both these elements can be calculated as follows:
The molar mass of water = 18.02 g
Mass
Mass
One can check the purity of a given sample by analyzing percentage composition.
Equivalent Weight:
Equivalent weight of any substance is that weight which react or liberate 1g of hydrogen (11.2 litre of H2 at STP) or 8g of oxygen or 35.5g of chlorine or 80g of bromine or 127g of iodine or 108g of silver or 1 mole of electron.
Equivalent weight is a number and when it is denoted in grams, it is called gram equivalent.
It depends upon the nature of the chemical reaction in which the substance takes part
How To Find Equivalent Weight:
Equivalent Weight
n-Factor or Valence Factor:
It calculates the molar ratio of the species taking part in reactions that are, reactants. The reciprocal of the n-factor 's ratio of the reactants represents the molar ratio of the reactants. For example, If A (having n-factor = a) reacts with B (having n-factor = b) then its n-factor's ratio is a: b, so the molar ratio of A to B is b: a.
It can be represented as follows:
Calculation of n-Factor
Before calculating the n-factor of any of the reactants in a given chemical reaction we must have a clear idea about the type of reaction. The reaction may be any of these types:
(i) Acid-base or neutralization reaction
(li) Redox reaction
Acid-Base or Neutralization Reactions:
As we know according to the Arrhenius concept, "An acid provides H+ ion(s) while a base provides OH- ion(s) in neutralization these H+ and OH- ion/ions combines together".
The number of H+ ion(s) and OH- ion(s) represent the n-factor for acid and base respectively, that is, basicity and acidity respectively.
Example,Redox Reactions
These reactions involve oxidation and reduction simultaneously. Here the exchange of electrons occurs. To find the n-factor for Oxidizing or agent we must find out the change in the oxidation state of these species.
You will be learning the following in detail in the chapter of redox. For now, just look at the definition. Sufficient questions will be practiced later.
For Redox Reactions:
E = (Molecular weight) / (Change in oxidation number),
x= change in oxidation state
For Example, for KMnO4
(a) In acidic medium: E = M/5
5 unit change in oxidation number.
(b) In basic medium: E = M/1
one unit change in oxidation number
(c) In neutral medium: E = M/3
3 unit change in oxidation number
Formulae for calculation of Equivalent Weight:
For Acids:
E = (Molecular weight) / (Protocity or Basicity of Acid), x= number of furnishable protons
For Example, for H3PO4, E = M/3
For H2SO4 , E =M/2For Bases:
E = (Molecular weight) / (Acidity or number of OH- ions),x= number of furnishable OH- ions
For Example, for Ca(OH)2, E = M/2
For Al(OH)3, E =M/3For Ions:
E = (Molecular weight) / (Charge on ion), x= charge on ion
For Example, for SO42-, E = M/2
For PO43-, E = M/3For Compounds:
E = (Molecular weight) / (total positive charge or negative charge present in compound),
x= total positive charge or negative charge present in the compound
For Example, for CaCO3, E = M/2
For AlCl3, E =M/3
For Acidic Salt:
E = (Molecular weight) / (Number of replaceable H-atoms)
For example, for H3PO4
Metal displacement method
E1 / E2 = W1 / W2
Also Read:
Recommended topic video on (Percent Composition Formula)
Some Solved Examples
Que 1: Find the equivalent mass of H3PO4 in the reaction:
1) 55
2) 43
3) 49
4) 37
Solution
In this given reaction only two hydrogen atoms are replaced so their equivalent mass will be given as follows:
Equivalent mass of H3PO4 = (Molecular Mass of H3PO4 ) / 2
=98 / 2
= 49
Hence, the answer is option (3).
Que 2: 74.5 g of metallic chloride contains 35.5 g of chlorine. The equivalent weight of the metal is
1) 19.5
2) 35.5
3) 39
4) 78
Solution
74.5 g of metallic chloride contains 35.5 g of chlorine.
Therefore, the weight of another element present in the chloride is
= Weight of Metal Chloride - Weight of Chloride
= 74.5 - 35.5 = 39 g
Thus, 39 g of the metal combines with 35.5 g of Chlorine
Mole of Chloride present = weight/molar mass = 35.5/35.5 = 1 mole
So, one mole of Cl is present and we know the charge on Cl is (-1).
So, MCl configuration will be there where M-metal.
MCl = M++ Cl-
So, one mole of Metal will be present and the charge would be (+1) on metal.
Molecular weight = mass of 1 mol metal = 39 g
Equivalent Weight = Molecular weight/ Valency.
Equivalent Weight = 39/1 =39
hence, the equivalent weight of the metal is 39.
Hence, the answer is the option (3).
Que 3: The oxide of a metal has 32% oxygen. Its equivalent weight would be
1) 17
2) 34
3) 32
4) 8
Solution
100g of the oxide contains 68g of metal and 32g of oxygen
Hence, the answer is the option (1).
Que 4: What is the equivalent weight of Sulphuric Acid (H2SO4) when it behaves as a diprotic acid?
1) 98
2) 49
3) 24.5
4) 12.25
Solution
n-factor of H2SO4 = 2
Hence, the answer is the option (2)
Que 5. A compound possesses 8% sulfur by mass. The least molecular mass is
1) 200
2) 400
3) 155
4) 355
Solution
Let, the molar mass of the compound be M.
The compound contains 8% by weight of Sulphur, mathematically it implies
Hence, the answer is the option (2).
Practice more Questions from the link given below:
Summary
Percentage composition is very important in chemistry because it helps in determining proportion of each element within a compound.This information is vital for understanding a compound's properties, identifying it, and calculating its empirical and molecular formulas.It’s also a analytical educational tool—serving as a bridge between chemical formulas and real-world applications. Its ability to authenticate purity and accuracy strengthens both lab precision and industrial credibility.
