Acids Bases and Salts - Definition, Types, Concept, FAQs
Have you ever wondered why some substances taste sour, some taste bitter, and some remain neutral? How do simple compounds like vinegar, baking soda, or common salt behave so differently in water? You will get these answers by reading this article on acids, bases and salts. Acids are known for their sour taste and their reaction with metals, bases are bitter and slippery, while salts are products of their neutralisation.
This Story also Contains
- Acid
- Bases
- Salts
- Salt is an Acid or Base
- Discovery of Acids, Bases and Salts
- Lux-Flood Concept
- Difference Between Acids, Bases and Salts
- Some Solved Examples
In this article, we will read about the acid-base and their discovery, concepts and some solved examples and reaction related to them.
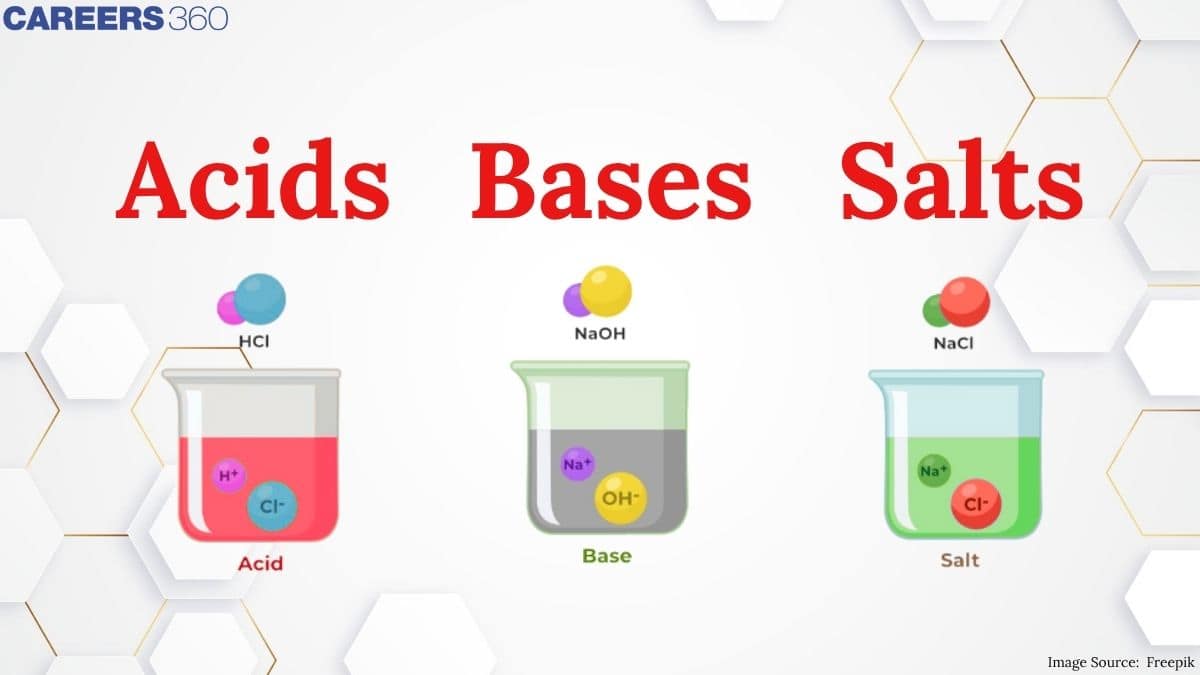
Acid
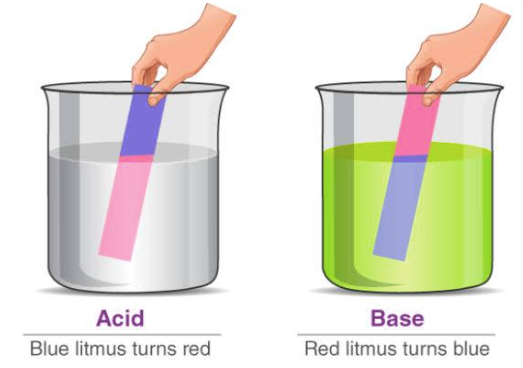
The word acid is derived from the word acidus meaning sour to taste. Acidic chemical compounds are known to give a sour or acidic taste when allowed to dissolve in water. An acid can remain energetically favourable even after loss of a hydrogen ion from its compound. Acids turn blue litmus red. The reason for this output s that the pigment in the litmus paper reacts with the H+ ions resulting in chemical changes where the chemical bonds are tuned to reflect light of longer wavelength making it appear red to our eyes. The reaction between the pigment in the litmus paper and the H+ ions of an acid leads to the absorption of blue to green wavelengths. Acids mainly oxidize other chemical compounds or simply change the colour of the substance. Acids can be either organic or inorganic, former containing a carboxyl group, a hydroxyl group and hydrogen atoms and the later contains a metal ion.
Bases
Bases are identified by their slippery texture and a bitter taste. Bases able to get dissolved in water are defined as alkali. Bases react with acids to produce salt and water and this chemical reaction is termed as neutralisation. Bases turn red litmus blue. The reason behind this colour change is explained as follows: The OH- ions or hydroxyl ions react differently with the pigment present in the litmus paper. It absorbs green and red wavelengths on reacting with the hydroxyl ion, reflecting short wavelengths to appear blue in colour. Bases are capable of changing the colour of the indicators. Phenolphthalein turns pink in the presence of a base.
Salts
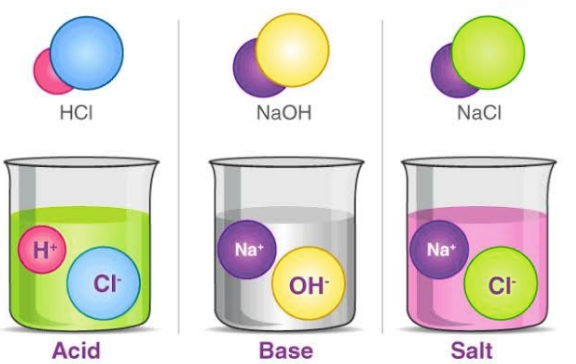
Salts are produced as a result of a reaction between an acid and a base i.e. neutralisation reaction. Salts are described as ionic compounds composed of a cation and an anion, where a cation is other than H+ and an anion is other than OH-.Salt is a chemical species that can make water less acidic or take away the acidity of water. The best-known example of a salt is NaCl (sodium chloride).Acid-base salt water example
Salt is an Acid or Base
Salts can be of the following types-
-
Acidic salt
-
Basic/Alkali salt
-
Double salt
-
Mixed salt
Discovery of Acids, Bases and Salts
In 1663, Robert Boyle noticed a class of substances having sour taste, ability to change colour of vegetable dyes (indicators), tendency to react with certain metals to evolve hydrogen and high solvent power. These substances were called acids. The term base, was introduced by Roulle in 1754. Bases are regarded as chemical substances changing red litmus blue, slippery in nature, soapy to touch, neutralize acids to form salt amd water, tasted bitter. Salts are nothing but ionic compounds formed by the reaction between acid and base giving out water molecule. Salts can be organic or inorganic. The relative amount of ions present in the salt makes it neutral. After the discovery of oxygen, Lavoisier (1778) suggested that oxygen is essential component of all acids. This was disproved by Dave (1816). He showed that hydrochloric acid does not contain oxygen. Further, Leibig in 1838 defined acids are the substances having hydrogen atom or atoms replaceable by metals. There afterwards, concepts like Arrhenius, Bronsted-Lowry, Lewis and Lux-Flood etc were put forward.
Let us see one by one:-
Arrhenius Concept
According to him acid is defined as a substance that dissociates to produce hydrogen ions when dissolved in water.
Eg: HCl, H2SO4, SO3
$\mathrm{HCl}_{\text {gas }}+$ Water $\rightarrow \mathrm{H}^{+}{ }_{\mathrm{aq}}+\mathrm{Cl}^{-}{ }_{\mathrm{aq}}$
A base is defined as a substance that dissociates in water to produce hydroxyl ions.
Eg: NaOH, KOH, NH3
|
Related Topics Link, |
Bronsted-Lowry Concept
J.N. Bronsted and T.M. Lowry independently forwarded the concept of acids and bases in 1923. They defined acids as follows:
Acids are proton donors.
Eg: $\mathrm{CH}_3 \mathrm{COOH} \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}+\mathrm{H}^{+}$
Bases are proton acceptors.
Eg: $\mathrm{CH}_3 \mathrm{COOH}+\mathrm{H}^{+} \rightleftharpoons \mathrm{CH}_3 \mathrm{COOH}$
From above example, it is possible to say that when an acid loses proton, the residual part of it has a tendency to regain proton. Hence, it acts as a base. Thus, an acid and a base maybe therefore, defined by the general equation-
Acid ⇌ H++ Base
Lewis Concept
G.N Lewis in 1923 defined acid as an electron pair acceptor and base as a electron pair donor.
Lux-Flood Concept
The definitions proposed by H. Lux (1939) were extended by H. Flood (1947). They described acid-base behaviour in terms of the oxide ion.
The base is an oxide ion donor, while the acid is an oxide ion acceptor.
$\begin{aligned} & \mathrm{CaO}+\mathrm{SiO}_2 \rightarrow \mathrm{CaSiO}_3 \\ & \mathrm{CaO} \rightarrow \mathrm{Ca}\end{aligned}$
This concept has been extended to include transfer of anions like halide (X-), sulphide(S2-).
The bases are anion donor while acids are anion acceptors.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 1 Some basic concepts of Chemistry
- NCERT notes Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
Is Salt Acidic or Basic?
Salts can be acidic, basic or neutral.
NaCl, KCl are neutral salts because these salts are formed out of a strong acid and a strong base, which do not hydrolyse. The pH remains constant, i.e. neutral at 7. The cation doesn’t alter the H+ ion concentration, and the anion does not attract the H+ ion. Hence, the salt remains neutral. Acidic salts are formed as a product of a neutralisation reaction between a strong acid and a weak base.
E.g. – ammonium chloride NH4Cl
Basic salts are a result of reaction between Weak acids and a strong base. E.g. – sodium acetate (NaAc).
Difference Between Acids, Bases and Salts
- An acid is described as a chemical compound which makes the water solution sour to taste and turns blue litmus to red colour.
- A base is a chemical compound whose aqueous solution is bitter to taste. It turns red litmus blue in colour or is capable of neutralising acids.
- Table Salt, being a neutral chemical species, has nil effect on the litmus paper.
- E.g.: NaCl (sodium salt) is obtained as a product of the reaction between a strong acid - Hydrochloric acid (HCl), and a strong base - sodium hydroxide (NaOH). NaCl is a cluster of Na+ cations and Cl- anions.
- Acids can be naturally occurring or synthetic. They are very important I the world of chemistry because they allow us to separate otherwise chemically similar substances.
- Natural acids are organic compounds that contain hydrogen and oxygen. They can be classified into either strong or weak acids based on their extent of ionisation.
- Some naturally occurring acids are: Vinegar (acetic acid), citric acid, tartaric acid.
Also check-
Some Solved Examples
Question 1: According to Brosted $\mathrm{H}_2 \mathrm{O}$ behaves like
1) Bronsted Acid
2) Bronsted Base
3) (correct) Amphoteric
4) None of these
Solution:
As we learned from
Dual nature of $\mathrm{H}_2 \mathrm{O}$ -
Water ( $\mathrm{H}_2 \mathrm{O}$ ) can play the role of an acid as well as base.
- wherein
$
\mathrm{HCl}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{Cl}^{-}
$
$\mathrm{H}_2 \mathrm{O}$ act as base
$
\mathrm{H}_2 \mathrm{O}+\mathrm{NH}_3 \rightleftharpoons \mathrm{NH}_4^{+}+\mathrm{O} \overline{\mathrm{H}}
$
$\mathrm{H}_2 \mathrm{O}$ act as acid
With strong acid $\mathrm{H}_2 \mathrm{O}$ will release $\mathrm{H}^{+}$and strong base $\mathrm{H}_2 \mathrm{O}$ will gain $\mathrm{H}^{+}$
Hence, the answer is option (3).
Question 2:
Conjugate base of H2O will be :
1) (correct) OH-
2) H3O+
3) O2-
4) None of these
Solution:
As we learned from
Conjugate acid-base pair -
The acid-base pair that differ only by one proton is called a conjugate acid-base pair.
Bronsted acid
$-\mathrm{H}^{+} \rightarrow$ Conjugate base
$$
\mathrm{H}_2 \mathrm{O}-\mathrm{H}^{+} \rightarrow \mathrm{OH}^{-}
$$
Hence, the answer is option (1).
Question 3:
Correct pair of Lewis acid and Lewis base respectively.
1) NH3 , BF3
2) NH3, H2O
3) BF3 , BCl3
4) (correct) BF3 , NH3
Solution:
As we learned
Lewis acids and bases -
Lewis defined an acid as a species that accepts an electron pair and a base that donates an electron.
- wherein
In Lewis acid, many acids do not have protons.
$
\begin{aligned}
& \text { e.g. } \mathrm{BF}_3 \\
& \mathrm{BF}_3+\mathrm{NH}_3 \rightarrow B F_3: \mathrm{NH}_3
\end{aligned}
$
Hence, the answer is option (4).
Practice More Question With The Link Given Below
| Bronsted-Lowry and Lewis Acid-Base theory practice questions and MCQs |
| Ionisation Constant of Acids and Bases and pH of strong Acids and Bases practice questions and MCQs |
Frequently Asked Questions (FAQs)
According to Arrhenius concept, acids are classified as the substances wich dissociates into H+ ions when dissolved in water.
Table salt is neutral.
acid turns blue litmus red.
Base turns red litmus blue.
There will be no change in the litmus paper colour when neutral salt is used. Blue litmus paper turns red when acidic salt is used.
Soap being basic in nature turns red litmus blue.
Acids react with base to form salt and water and this reaction is called neutralization reaction.
