Nature and Characteristics of Matter: What is, Examples, Particles
Chemistry explores what materials are made of, how their structures influence their properties, and how they transform under varying conditions. It bridges the physical and life sciences, offering insights into processes like cooking, digestion, metallurgy, and drug formulation. By disclosing how substances react and change, chemistry plays an essential role in day to day life—from developing new materials and medicines to understanding environmental or surrounding phenomena. As a core scientific discipline, it deepens our grasp of the natural world and supports advances across medicine, engineering, agriculture, and more.
This Story also Contains
- Importance of Chemistry And Nature of Matter
- Some Solved Examples
- Practice more Questions from the link given below:
- Conclusion
Importance of Chemistry And Nature of Matter
Chemistry is the branch of science that studies the composition, structure, and properties of matter. It explains these aspects through atoms and molecules, the fundamental building blocks of all substances. This is why chemistry is often referred to as the science of atoms and molecules. It plays an important role across scientific disciplines, linking closely with physics, biology, geology, and more. In everyday life, chemistry helps us understand natural processes like weather and brain function and supports technological advances such as computer operations. Chemical industries contribute very much to the economy by producing essential materials like fertilizers, acids, salts, dyes, polymers, medicines, soaps, and metals. Chemistry is also crucial for directing human needs in food production, healthcare, and the development of materials that enhance the quality of life.
Anything which has mass and occupies some space is called matter. Based on shape, size, and volume, the matter can be classified into solids. liquids and gases.
-
Solids have definite volume and definite shape.
-
Liquids have a definite volume but no definite shape. They take the shape of the container in which they are placed.
-
Gases have neither a definite volume nor a definite shape. They completely occupy the container in which they are placed.
These three states of matter are interconvertible by changing the conditions of temperature and pressure.
Solid
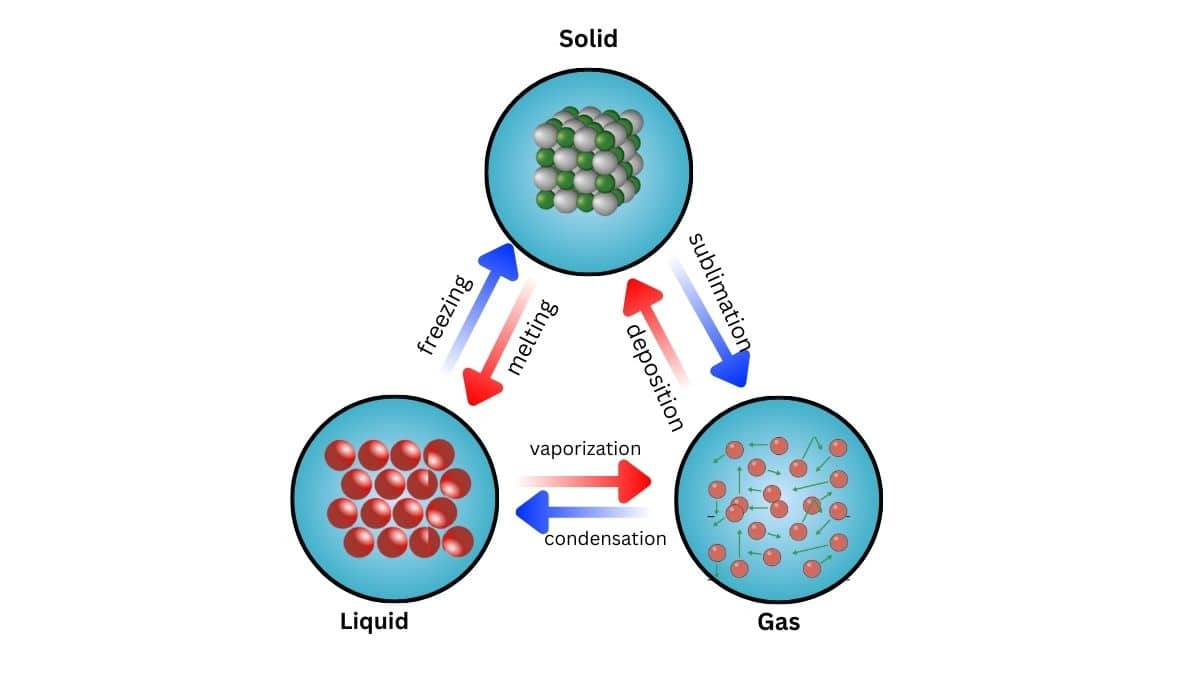
On heating, a solid usually changes to a liquid, and the liquid on further heating changes to the gaseous ( or vapour) state. In the reverse process, a gas on cooling liquifies to the liquid, and the liquid on further cooling freezes to the solid.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Recommended topic video on(Nature and Characteristics of Matter)
Some Solved Examples
Example 1: Which of the following is known as dry ice?
1) Gaseous CO2
2) Solid SiO2
3) CH4
4) Solid CO2
Solution
Solid CO2 is called as dry ice.
Hence, the answer is the option (4).
Example 2: Which of the following exists as a solid at room temperature?
1)Cl2
2)F2
3) I2
4) Br2
Solution
F2 and Cl2 are gases, Br2 is a liquid and I2 exists as a solid at room temperature because of the difference in intermolecular forces that act on between individual molecules.
Iodine is present in solid form at room temperature, while chlorine is a gas at room temperature because of the difference in intermolecular forces that act between individual molecules.
Hence, the answer is an option (3).
Example 3:What is the state of water below 0oC?
1) Solid
2) Liquid
3) Gas
4) Plasma
Solution
Water exists in three different forms i.e., solid, liquid, and gas. Below 0oC, intermolecular hydrogen bonding is very strong between the different water molecules, thus water exists in solid form as ice.
Hence, the answer is the option (1).
Practice more Questions from the link given below:
Conclusion
Our surroundings and environment consist of numerous substances, blazing human curiosity from ancient times. Science has helped structure that curiosity, enabling us to explore and explain the natural world. Chemistry plays a vital role in turning raw materials into essential products—fertilizers, acids, alkalis, dyes, and advanced organic and inorganic materials—driving both industrial growth and day to day well-being. By powering innovation and boosting economic activity, these chemical industries contribute significantly to national development and enhance quality of life.
Frequently Asked Questions (FAQs)
Matter are classified in 2 ways
AZT (azidothymidine)
Limiting the definition simply to something that has mass and occupies space will suffice as the definition of matter.
Antipyretics drugs are use to decrease the high temp.
Cisplatin and taxol
