Introduction to P Block Elements - Introduction, Properties & Applications, FAQs
Introduction to p-Block Elements
The last electron in a p block element occupies one of three p-orbitals of the corresponding orbits. There are six groups of p-block elements because a p-subshell has three degenerate p-orbitals that can accommodate two electrons. Due to their tendency to lose one electron, p-block elements are lustrous and usually good conductors of electricity and heat. In a p-block element like gallium, you'll find several astonishing properties of elements. Silicon is also a key component of glass, making it one of the most important metalloids in the p-block group.
Also read -
What are Introduction to p-Block Elements?
The element in which the last electron enters the outermost p-subshell is known as a p-block element. In the periodic table, the p block begins with the 13th group and ends with the 18th group.
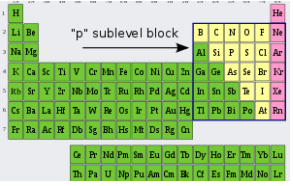
Some metals, non-metals, and metalloids are among these elements. Normal or representative elements are made up of s-block and p-block components (except zero group elements). Each period of the periodic table concludes with a noble gas with a closed shell ns2np6 structure, which is a member of the zero group (18th group). There are two chemically significant non-metal groups before the noble gas group.
Also read :
The periodic table blocks:
The elements in the periodic table are divided into blocks based on their valence electron orbitals. Four blocks of the periodic table are S-block, p-block, d-block, and f-block. If a new element is detected, it will be added to the g-block. Each block denotes the electron sublevel that is currently being filled.
The main group (s- and p-blocks), transition metal (d-block), and inner transition metal (f-block) elements are all based on the four periodic table blocks.
The s-block:
Except for helium, the s-block elements are on the periodic table's left side.
All of the s-block elements are metals, with the exception of helium (and possibly hydrogen). Alkali metals and alkaline earth metals make up the s-block.
Soft solids with low melting points are common for S-block elements.
All s-block elements are electropositive and reactive, with the exception of helium.
The p-block:
The p-block elements are found on the periodic table's right side. They are the table's final six element groups (except for helium).
All nonmetals (excluding hydrogen and helium), metalloids, and post-transition metals are classified as P-block elements.
The valence electrons of P-block elements can be gained, lost, or shared.
Covalent compounds are formed by the majority of p-block components.
The d-block:
D-block elements constitutes the transition metals (groups 3-12).
The properties of D-block elements are similar to those of highly reactive electropositive s-block elements and more electronegative p-block elements. It is for this reason that they are referred to as "transition" metals.
All of these elements are metals that have two or more oxidation states.
The melting and boiling points of D-block elements are usually quite high.
The f-block:
Lanthanides and actinides comprises f-block elements, or inner transition metals. They are the two rows of elements below the periodic table's main body.
The oxidation states of F-block elements are varied.
The melting values of most f-block elements are extremely high.
Colored complexes and salts are generated by these elements, but they are paler than those formed by d-block elements.
Properties of Introduction to p-Block Elements:
The last electron of the p-block components reaches the p-subshell of the outermost shell.
The np subshell eventually fills up in these elements. From ns2 np1 to ns2 np6, the valence shell configuration evolves.
The electrical configuration of the introduction to p-block elements is ns2np1-6 in general (Except Helium).
The number of electrons in the p-block element's penultimate shell is either 2 or 8 or 18.
p-block elements, with the exception of F and inert gases, have a range of oxidation states ranging from +n to (n- 8), where n is the number of electrons in the outermost shell.
Higher members of the p-block can show electrovalency, but the p-block parts in general show covalency. Electrovalency is demonstrated by highly electronegative elements like halogens F, Cl, and others absorbing electrons and producing anions. Some of the components.
There is a consistent rise in non-metallic nature from left to right. Non-metallic character, on the other hand, decreases from top to bottom in the groups.
In a period, ionisation energies increase from left to right, while in a group, they drop from top to bottom. Due to half-filled and fully-filled ionisation energies, members of groups VA and zero have very high values.
Reducing nature declines from left to right in every cycle, whereas oxidising nature grows. From top to bottom, reducing nature grows in a group. Halogens are powerful oxidizers.
Acidic oxides are formed by the majority of the p-block elements.
The flame is not coloured by any member of the p-block series or the salts.
The phenomena of allotropy can be seen in a variety of p-block series elements. This feature can be found in carbon, silicon, phosphorus, sulphur, boron, germanium, tin, arsenic, and other elements.
Many elements in the p-block series, including as carbon, silicon, germanium, nitrogen, oxygen, and sulphur, exhibit the catenation feature.
NCERT Chemistry Notes:
Applications of Introduction to the p-block elements:
Borax, a boron-based chemical, is used in the glass and pottery industries.
Boron is also utilised in soap and detergent manufacturing.
Boron is utilised in bullet-proof jackets and aircraft.
Boron is added to steel to make it harder.
Aluminum is utilised in kitchenware, coils, wires, iron and zinc protection, and wrapping foils. It can also be used as a reducing agent.
Semiconductors include germanium, arsenic, silicon, and gallium.
Iodine tincture contains iodine.
Disinfectants contain chlorine.
Carbon and its compounds are employed in a variety of applications.
Also check-

