NH4OH Full Form
What is Full Form of NH4OH ?
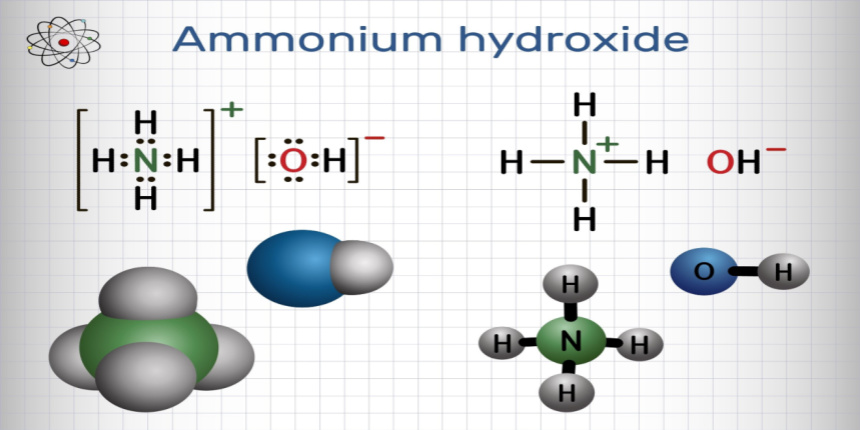
NH4OH is also known as Aqueous Ammonia, Aqua Ammonia, Ammonium Hydroxide or Ammonia Liquor. It has a strong odor and is a colorless liquid. Furthermore, dilute ammonium hydroxide is effective as a cleaner. The solution consists of water and ammonia with minor quantities of the ammonium ion, and the hydroxide ion. These ions are bound together by ionic bonding.
Physical Properties of Ammonium Hydroxide
Molar mass = 35.04 g/mol
Density = 0.91 g/mL
Melting Point = -57.5ºC
Boiling point = 37ºC
Highly miscible in water
Ammonium hydroxide is a weak compound that partially dissociates in the water while maintaining equilibrium between the ammonium ion and the hydroxide ion.
The equilibrium is used to regulate the pH of solutions since the ion OH- maximizes the pH of any solution's basicity level.
Saturated Solutions of Ammonium Hydroxide
Ammonia solubility falls as the temperature of the aqueous solvent rises. The behaviour of ammonia is quite similar to that of other gases. The density of an ammonia solution in water decreases as the quantity of dissolved ammonia increases.
At 288.75 K, the density of a saturated ammonium hydroxide solution is 0.88 g per mole. This saturated ammonium hydroxide solution would contain roughly 35.6% ammonia by mass or 308 g of ammonia per liter of saturated ammonium hydroxide solution.
Uses
Ammonium hydroxide solution is a critical component in the production of chemical fertilizers. In these fertilizers, it is applied as a solution or as salt.
Ammonium hydroxide is used in the manufacturing of nitrogen-containing organic and inorganic compounds. It is the primary chemical used in the production of nitric acid.
Cleaning chemicals, such as window cleaning solvents, include a 1-3% solution of ammonium hydroxide. A solution of ammonia in water (scented or unscented) is also available as a cleaning agent.
Ammonia may be used to make chloramine, which is an excellent disinfectant.
Tannic acid-containing wood can be sealed in a container with an ammonium hydroxide solution to give it a dark-stained appearance. As a result, ammonium hydroxide solution is used to darken furniture.
Hazards
Ammonium Hydroxide is particularly hazardous in skin contact, eye contact, ingesting, and so on. Ammonium Hydroxide can cause burns when it comes into contact with the skin. Inhaling the spray mist might induce severe respiratory tract irritation, coughing, choking, or shortness of breath. Excessive overexposure will result in death. Redness, swelling, and itching are symptoms of ocular inflammation. Skin inflammation is characterized by swelling, scaling, reddening, and, in rare cases, blistering.
In the event of skin contact, wash the skin thoroughly with lots of water for at least 15 minutes while removing soiled clothes and shoes. On the inflamed face, apply an emollient. Before reusing clothes, they should be disinfected with hot water. Before you change your shoes, thoroughly clean them. Seek medical assistance right away.
